5,5‘‘‘-bis(aminoethyl)-2,2‘:5‘,2‘‘:5‘‘,2‘‘‘-quaterthiophene lead bromide
Chemical Formula: C20H22N2S4PbBr4
IUPAC: 5,5‘‘‘-bis(aminoethyl)-2,2‘:5‘,2‘‘:5‘‘,2‘‘‘-quaterthiophene lead(II) bromide
Alternate Names: AE4TPbBr4, (AEQT)PbBr4, AEQTPbBr4, 5,5‘‘‘-bis(aminoethyl)-2,2‘:5‘,2‘‘:5‘‘,2‘‘‘-quaterthiophene tetrabromoplumbate(II)
Organic: C20H22N2S4
Inorganic: PbBr4, Lead bromide
Dimensionality: 2D n: 1
Formal Stoichiometry:
C : 20
,
H : 22
,
N : 2
,
S : 4
,
Pb : 1
,
Br : 4
Atomic structure Verified
See all entries for this property (4 total)
Origin: experimental (T = 298.0 K)
Space group: C 2/c
Crystal system: monoclinic
| a: | 39.741 (±0.002) Å |
| b: | 5.842 (±0.003) Å |
| c: | 11.5734 (±0.0006) Å |
| α: | 90° |
| β: | 92.36 (±0.01)° |
| γ: | 90° |
- temperature = 298.0 K
Sample type: single crystal
Starting materials: AEQT.2HBr, PbBr2, ethylene glycol, HBr (48% in water)
Product: Yellow (AEQT)PbBr4 crystals
Description: Prepare the starting AEQT.2HBr salt using a technique similar to that described in detail for the synthesis of AMQT.2HCl [1]. Grow (AEQT)PbBr4 crystals from a slowly cooled, saturated, aqueous solution containing the organic and inorganic salts. First, weigh 14.5 mg (0.025 mmol) of AEQT.2HBr and 18.3 mg (0.050 mmol) of PbBr2 and add to a test tube under an inert atmosphere. Dissolve the contents in the sealed tube at 120 °C in a solvent mixture of 22 mL of deionized water, 1 mL of ethylene glycol, and 2 drops of 48% aqueous HBr, forming a nominally saturated yellow solution. Slow cool at 2 °C/h to 0 °C, to form small, yellow, sheetlike crystals of the desired (AEQT)PbBr4 compound. To prevent deforming the thin crystals, remove the product from the reaction tube using a pipet and deposit on filter paper to absorb the solution.
Comment: References: [1] (a) Muguruma, H.; Saito, T.; Sasaki, S.; Hotta, S.; Karube, I. J. Heterocycl. Chem. 1996, 33, 173. (b) Muguruma, H.; Saito, T.; Hiratsuka, A.; Karube, I.; Hotta, S. Langmuir 1996, 12, 5451.
Method: Single-crystal X-ray diffraction
Description: An (AEQT)PbBr4 crystal, with the approximate dimensions 0.01 mm X 0.27 mm X 0.30 mm, was selected under a microscope and attached to the end of a quartz fiber with 5 min epoxy. A full sphere of data was collected at room temperature on a Bruker SMART CCD diffractometer, equipped with a normal focus 2.4 kW sealed tube X-ray source (Mo Ka radiation). Refer to Page 6247 for details.
Comment: Refer to Table 2 for Positional and Thermal Parameters.
Entry added on: April 15, 2019, 9:54 p.m.
Entry added by: Xiaochen Du Duke University
Last updated on: June 16, 2022, 6:37 a.m.
Last updated by: Jannik Eisenlohr Michigan State University
Data correctness verified by:
- Rayan C Duke University
Download data
Band structure Verified
See all entries for this property (3 total)
Experimental then geometry optimized
Origin: computational
Crystal system:
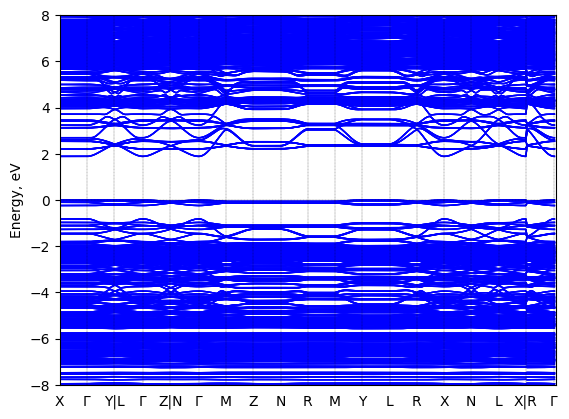
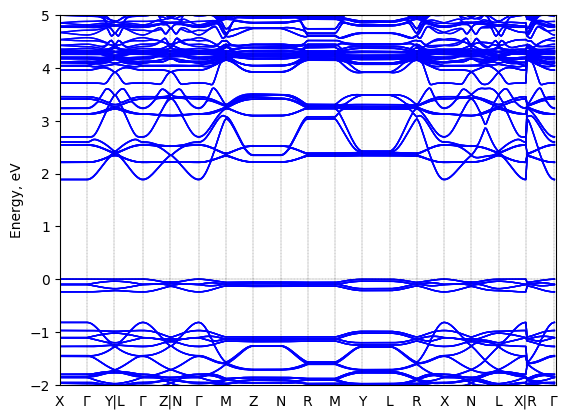
Sample type: single crystal
Code: FHI-aims
Level of theory: density functional theory
Exchange-correlation functional: HSE06 α = 0.25, ω = 0.11/bohr
K-point grid: 3x3x3
Level of relativity: atomic ZORA with spin-orbit coupling
Basis set definition: tight
Entry added on: May 8, 2019, 4:01 p.m.
Entry added by: Xiaochen Du Duke University
Last updated on: March 23, 2022, 5:59 p.m.
Last updated by: Rayan C Duke University
Data correctness verified by:
- Rayan C Duke University
Download data
Absorption spectrum Verified
Origin: experimental (T = 298.0 K)
Sample type: film
Starting materials: (AEQT)PbBr4, quartz, acetone, ethanol
Product: (AEQT)PbBr4 thin film. Film thickness between 500 Å and 900 Å.
Description: To prepare (AEQT)PbBr4, sonicate quartz samples in 2% (w/v) detergent solution in water (20 min), then sonicate in acetone (20 min) and ethanol (20 min). Boil in ethanol (5 min) and place in a 130 °C oven to dry. Add 0.003 g of (AEQT)PbBr4 compounds to 0.1 mL of methanol, and sonicate again for 10 min. Place the charge dropwise via a syringe on the tantalum heater of the SSTA chamber. Close chamber and evacuate all solvent with a rotary mechanical pump. Switch on a turbomolecular pump, and pump system to approximately 10^{-7} Torr. To initiate the evaporation, pass a large current of approximately 65 A through the heater for about 4 s. Anneal the (AEQT)PbBr4 films at 180 °C for 15 min.
Comment: Single source thermal ablation (SSTA) technique.
Method: UV-Vis absorption
Description: Absorption spectra were obtained at room temperature on the thermally ablated films using a Hewlett-Packard UV-vis 8543 spectrophotometer.
Comment: Refer to Figure 8.
Entry added on: May 14, 2020, 10:54 a.m.
Entry added by: Xiaochen Du Duke University
Last updated on: April 1, 2022, 12:43 p.m.
Last updated by: Rayan C Duke University
Data correctness verified by:
- Rayan C Duke University
Download data
Photoluminescence
See all entries for this property (3 total)
Origin: experimental (T = 298.0 K)
Sample type: film
Starting materials: (AEQT)PbBr4, quartz, acetone, ethanol
Product: (AEQT)PbBr4 thin film. Film thickness between 500 Å and 900 Å.
Description: To prepare (AEQT)PbBr4, sonicate quartz samples in 2% (w/v) detergent solution in water (20 min), then sonicate in acetone (20 min) and ethanol (20 min). Boil in ethanol (5 min) and place in a 130 °C oven to dry. Add 0.003 g of (AEQT)PbBr4 compounds to 0.1 mL of methanol, and sonicate again for 10 min. Place the charge dropwise via a syringe on the tantalum heater of the SSTA chamber. Close chamber and evacuate all solvent with a rotary mechanical pump. Switch on a turbomolecular pump, and pump system to approximately 10^{-7} Torr. To initiate the evaporation, pass a large current of approximately 65 A through the heater for about 4 s. Anneal the (AEQT)PbBr4 films at 180 °C for 15 min.
Comment: Single source thermal ablation (SSTA) technique.
Method: Photoluminescence
Description: Photoluminescence spectrum was recorded at room temperature using a Spex Fluorolog-2 spectrofluorometer. 370 nm light from a xenon arc lamp was used as the excitation source, after being passed through a SPEX 1680 0.22 m double monochromator. The emission was passed through a similar monochromator and detected with a SPEX 1911F photomultiplier tube (PMT).
Entry added on: March 23, 2022, 5:55 p.m.
Entry added by: Rayan C Duke University
Last updated on: April 1, 2022, 4:49 p.m.
Last updated by: Rayan C Duke University
Download data