1-(1-naphthyl)ethylammonium lead bromide
Chemical Formula: C24H28N2PbBr4
IUPAC: 1-(1-naphthyl)ethanaminium lead (II) bromide
Alternate Names: NEA2PbBr4, 1-1-NEA2PbBr4, racemic-NEA2PbBr4, 1-(1-naphthyl)ethylammonium tetrabromoplumbate(II)
Organic: C12H14N
Inorganic: PbBr4, Lead bromide
Dimensionality: 2D n: 1
Formal Stoichiometry:
C : 24
,
H : 28
,
N : 2
,
Pb : 1
,
Br : 4
Atomic structure Verified
See all entries for this property (3 total)
2D chiral perovskite
Origin: experimental (T = 298.0 K)
Space group: P 2₁
Crystal system: monoclinic
| a: | 19.2528 (±0.0009) Å |
| b: | 8.0769 (±0.0004) Å |
| c: | 8.728 (±0.0005) Å |
| α: | 90° |
| β: | 90.281 (±0.003)° |
| γ: | 90° |
- temperature = 298.0 K
Sample type: single crystal
Starting materials: 1-(1-naphthyl)ethylamine (98%), PbBr2
Product: 1-(1-naphthyl)ethylammoium lead bromide
Description: A hot solution of 1-(1-naphthyl)ethylamine (39 µL, 0.24 mmol ) and PbBr2 (45 mg, 0.12 mmol) in 0.5 ml of aq. HBr and 1.2 ml methanol is cooled from 95 °C to room temperature over 48 hr. The colorless plate-like crystals were filtered, washed with diethyl ether, and vacuum-dried.
Method: Single crystal X-ray diffraction
Description: Single crystal X-ray diffraction (XRD) was performed at 298 K on a Bruker APEX II CCD diffractometer using Mo-Kα radiation (λ=0.710 Å) and X-ray tube operating at 50 kV and 30 mA
Entry added on: Aug. 4, 2020, 2:37 p.m.
Entry added by: Manoj Kumar Jana Duke University
Last updated on: Aug. 22, 2022, 3:31 p.m.
Last updated by: Rayan C Duke University
Data correctness verified by:
- Rayan C Duke University
Download data
Band structure Verified
2D chiral perovskite
Origin: computational
Crystal system: monoclinic
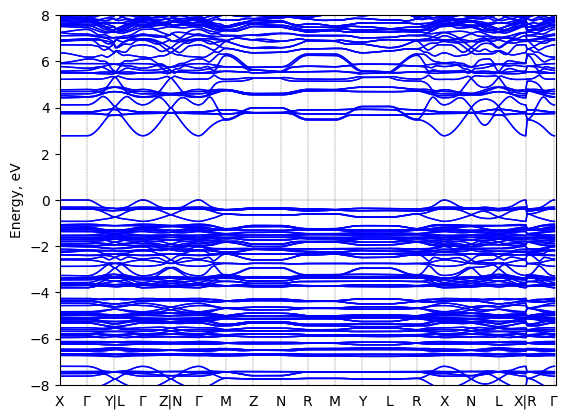
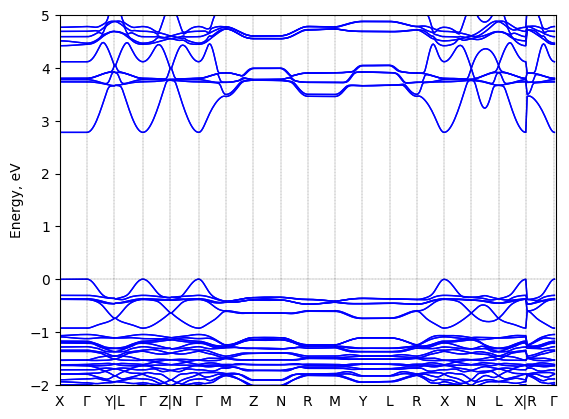
Sample type: single crystal
Code: FHI-aims
Level of theory: DFT
Exchange-correlation functional: HSE06
K-point grid: 3×4×4
Level of relativity: atomic ZORA with SOC
Basis set definition: NAO
Geometry used in the calculation
External repositories:
Entry added on: Aug. 12, 2020, 2:47 p.m.
Entry added by: Ruyi Song Chemistry department, Duke university
Last updated on: Sept. 16, 2022, 8:24 a.m.
Last updated by: Rayan C Duke University
Data correctness verified by:
- Rayan C Duke University
Download data
DOI for this data set: 10.6084/m9.figshare.12797498
Data set ID: 1634 Did you find any mistakes or inconsistencies about this data? Send us a note and we'll have a look at it and send you a reply. Thanks!Melting Point
Melting temperature = 221°C
Origin: experimental
Space group: P21c
Sample type: bulk polycrystalline
Starting materials: 1-(1-naphthyl) ethylamine (98%, Sigma Aldrich), lead bromide (PbBr2, 99.99%, TCI chemicals) and hydrobromic acid (HBr) (48 wt% in H2O, >99.99%, Sigma Aldrich)
Product: 1-(1-naphthyl)ethylammonium]2PbBr4
Description: To grow rac-NPB perovskite crystals, stoichiometric amounts of PbBr2 (90 mg, 0.24 mmol) and 1-(1-naphthyl) ethylamine (78 µL, 0.48 mmol) were dissolved in aq. HBr (1.0 mL) and methanol (2.4 mL) in a sealed vial at 95 °C. The hot solution was slowly cooled to room temperature (21 °C) over a period of 24 h in a water bath, resulting in the formation of layered flakes of transparent rac-NPB single crystals.
Method: Differential Scanning Calorimetry
Description: Differential Scanning Calorimetry: DSC measurements were performed using a TA Discovery DSC instrument using various ramping rates and temperature ranges (as described in the main text) using a hermetically sealed aluminum pan and lid. Prior to experiments, the DSC setup was calibrated with metallic indium (melting temperature: 156.6 °C; enthalpy of melting: 28.71 J g−1 ), which upon repeating the experiment showed an acceptable temperature offset of 0.2 °C and melting enthalpy offset of 0.04%. Calibration and the above measurement were carried out at a ramp rate of 5 °C min−1 . DSC analyses of crystalline S-NPB and rac-NPB perovskites were carried out by hermetically sealing corresponding crystals (≈5.0 mg) in aluminum pan/lid, and ramping temperature from 25 to 250 °C at a ramp rate of 5 °C min−1.
Entry added on: March 8, 2021, 12:43 a.m.
Entry added by: Akash Singh
Last updated on: Aug. 28, 2022, 5:35 p.m.
Last updated by: Rayan C Duke University
Download data
Thermogravimetric behavior
Degradation onset temperature = 215.0 °C
Origin: experimental
Space group: P21c
Sample type: bulk polycrystalline
Starting materials: 1-(1-naphthyl) ethylamine (98%, Sigma Aldrich), lead bromide (PbBr2, 99.99%, TCI chemicals) and hydrobromic acid (HBr) (48 wt% in H2O, >99.99%, Sigma Aldrich)
Product: 1-(1-naphthyl)ethylammonium]2PbBr4
Description: To grow rac-NPB perovskite crystals, stoichiometric amounts of PbBr2 (90 mg, 0.24 mmol) and 1-(1-naphthyl) ethylamine (78 µL, 0.48 mmol) were dissolved in aq. HBr (1.0 mL) and methanol (2.4 mL) in a sealed vial at 95 °C. The hot solution was slowly cooled to room temperature (21 °C) over a period of 24 h in a water bath, resulting in the formation of layered flakes of transparent rac-NPB single crystals.
Method: Thermogravimetric Analysis
Description: Thermogravimetric Analysis: TGA measurements were performed on a TA Q50 instrument using a 5 °C min−1 ramping rate from 25 to 300 °C under nitrogen gas flow (40 mL min−1) with samples (≈4.5 mg) of single crystals of S-NPB and rac-NPB perovskite.
Entry added on: March 8, 2021, 12:52 a.m.
Entry added by: Akash Singh
Last updated on: March 28, 2023, 8:32 a.m.
Last updated by: Rayan C Duke University
Download data