S-2-methylbutylammonium lead bromide
Chemical Formula: C10H28N2PbBr4
IUPAC: S-2-methylbutylammonium lead (II) Bromide
Alternate Names: (S-2-MeBA)2PbBr4
Organic: C5H14N
Inorganic: PbBr4, lead bromide
Dimensionality: 2D n: 1
Formal Stoichiometry:
C : 10
,
H : 28
,
N : 2
,
Pb : 1
,
Br : 4
Atomic structure
See all entries for this property (2 total)
Origin: experimental (T = 100.0 K)
Space group: P 2₁
Crystal system: monoclinic
| a: | 15.31429958 Å |
| b: | 8.220509529 Å |
| c: | 8.172200203 Å |
| α: | 90° |
| β: | 100.7149963° |
| γ: | 90° |
- temperature = 100.0 K
Sample type: single crystal
Starting materials: S-2-MeBA, PbBr2, HI, dimethylformamide (DMF), dichloromethane (DCM), ethyl ether
Product: yellow flaky single crystals (S-2-MeBA)2PbBr4
Description: Add (S-2-MeBA) (0.25 mmol) and PbBr2 (0.125 mmol) to 0.8 mL of an aqueous HI solution. Obtain a clear solution at 95 degrees Celsius, then cool to room temperature at a rate of 2 degrees Celsius per hour. The cooling process yields about 0.07 grams of (S-2-MeBA)2PbBr4 crystals. Then, dissolve 0.03 g of the obtained crystals in dimethylformamide (DMF) (0.8 mol/L) in a small uncovered vial, and place it in a larger vial holding dichloromethane (DCM). Over a week-long period, the DCM vapors diffuse into the vial with the DMF solution and produce yellow flaky single crystals ((S-2-MeBA)2PbBr4) as well as S-2-MeBA salt, light-yellow needle-shaped crystals. The crystals were filtered, washed with ethyl ether, and vacuum-dried. The (S-2-MeBA)2PbBr4 crystals can be separated from the salt crystals under a microscope, and they were then used for single-crystal X-ray diffraction (SC-XRD).
Comment: Differential scanning calorimetry (DSC): A TA Discovery DSC 2500 was used to carry out low-temperature conventional differential scanning calorimetry (DSC) measurements. The instrument was under helium purge with a 5 K/min ramp from 298 K to 124 K and back to 298 K (with use of an LN2P cooler). Sample powder was loaded into a Tzero Hermetic aluminum pan, with temperature and enthalpy calibrated in advance. A similar instrument was used to perform high-temperature DSC measurements under nitrogen purge. The ramp rate was 5 K/min from 298 K to 603 K. An aluminum pan was hermetically sealed to contain samples.
Method: Differential scanning calorimetry (DSC)
Description: A TA Discovery DSC 2500 was used to carry out low-temperature conventional differential scanning calorimetry (DSC) measurements. The instrument was under helium purge with a 5 K/min ramp from 298 K to 124 K and back to 298 K (with use of an LN2P cooler). Sample powder was loaded into a Tzero Hermetic aluminum pan, with temperature and enthalpy calibrated in advance. A similar instrument was used to perform high-temperature DSC measurements under nitrogen purge. The ramp rate was 5 K/min from 298 K to 603 K. An aluminum pan was hermetically sealed to contain samples.
Entry added by: Ruyi Song Chemistry department, Duke university
Last updated on: Sept. 20, 2022, 10:52 a.m.
Last updated by: Harrison York Duke University
Download data
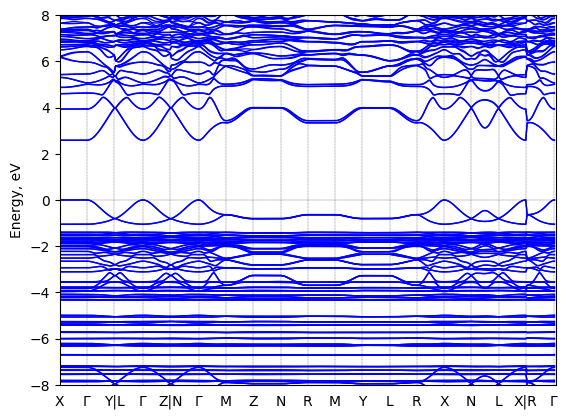
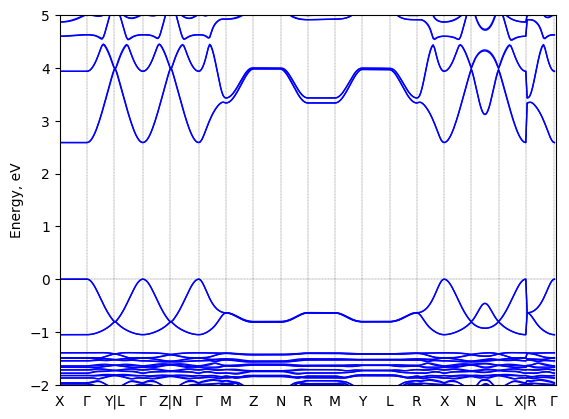
Band structure
See all entries for this property (2 total)
T = 298.0 K
Origin: experimental
Space group: P 2₁
Crystal system: monoclinic
Sample type: single crystal
Starting materials: S-2-MeBA, PbBr2, HI, dimethylformamide (DMF), dichloromethane (DCM), ethyl ether
Product: yellow flaky single crystals (S-2-MeBA)2PbBr4
Description: Add (S-2-MeBA) (0.25 mmol) and PbBr2 (0.125 mmol) to 0.8 mL of an aqueous HI solution. Obtain a clear solution at 95 degrees Celsius, then cool to room temperature at a rate of 2 degrees Celsius per hour. The cooling process yields about 0.07 grams of (S-2-MeBA)2PbBr4 crystals. Then, dissolve 0.03 g of the obtained crystals in dimethylformamide (DMF) (0.8 mol/L) in a small uncovered vial, and place it in a larger vial holding dichloromethane (DCM). Over a week-long period, the DCM vapors diffuse into the vial with the DMF solution and produce yellow flaky single crystals ((S-2-MeBA)2PbBr4) as well as S-2-MeBA salt, light-yellow needle-shaped crystals. The crystals were filtered, washed with ethyl ether, and vacuum-dried. The (S-2-MeBA)2PbBr4 crystals can be separated from the salt crystals under a microscope, and they were then used for single-crystal X-ray diffraction (SC-XRD).
Code: FHI-aims
Level of theory: density functional theory
Exchange-correlation functional: HSE06 [α=0.25, ω=0.11 Å^(-1)]
K-point grid: 3x4x4
Level of relativity: relativistic atomic ZORA scalar, include_spin_orbit
Basis set definition: FHI-aims intermediate settings
Numerical accuracy: FHI-aims intermediate settings
Entry added on: Oct. 3, 2022, 11:18 p.m.
Entry added by: Harrison York Duke University
Last updated on: Oct. 6, 2022, 6:36 p.m.
Last updated by: Harrison York Duke University
Download data