Y. Xie, R. Song, A. Singh, M. Jana, V. Blum, and D. Mitzi, Kinetically Controlled Structural Transitions in Layered Halide-Based Perovskites: An Approach to Modulate Spin Splitting, Journal of the American Chemical Society 144 (33), 15223‑15235 (2022). doi: https://doi.org/10.1021/jacs.2c05574.
S-2-methylbutylammonium lead iodide: atomic structure
See all entries for this property (6 total)
Origin: experimental (T = 298.0 K)
Space group: P 2₁
Crystal system: monoclinic
| a: | 30.99020004 Å |
| b: | 8.777999878 Å |
| c: | 8.814900398 Å |
| α: | 90° |
| β: | 90° |
| γ: | 103.375° |
- temperature = 298.0 K
Sample type: single crystal
Starting materials: S-2-MeBA, PbI2, HI, dimethylformamide (DMF), dichloromethane (DCM), ethyl ether
Product: yellow flaky single crystals (S-2-MeBA)2PbI4
Description: Add (S-2-MeBA) (0.25 mmol) and PbI2 (0.125 mmol) to 0.8 mL of an aqueous HI solution. Obtain a clear solution at 95 degrees Celsius, then cool to room temperature at a rate of 2 degrees Celsius per hour. The cooling process yields about 0.07 grams of (S-2-MeBA)2PbI4 crystals. Then, dissolve 0.03 g of the obtained crystals in dimethylformamide (DMF) (0.8 mol/L) in a small uncovered vial, and place it in a larger vial holding dichloromethane (DCM). Over a week-long period, the DCM vapors diffuse into the vial with the DMF solution and produce yellow flaky single crystals ((S-2-MeBA)2PbI4) as well as S-2-MeBA salt, light-yellow needle-shaped crystals. The crystals were filtered, washed with ethyl ether, and vacuum-dried. The (S-2-MeBA)2PbI4 crystals can be separated from the salt crystals under a microscope, and they were then used for single-crystal X-ray diffraction (SC-XRD).
Method: Single-crystal X-ray diffraction (SC-XRD)
Description: A Rigaku XtaLAB Synergy-S diffractometer using Mo Kα radiation (λ = 0.71073 Å) and functioning at 50 kV and 30 mA gathered data on (S-2-MeBA)2PbI4 crystals at room temperature (298 K). Data was also collected at 100 K, incorporating an 800 Series Cryostream Cooler. CrysAlisPro performed peak hunting, data reduction, and numerical absorption correction. SHELXS direct methods and SHELXL least-squares method solved crystal structures. PLATON’s ADDSYM tool analyzed the symmetry of full and isolated inorganic structures (default tolerance values and distance criteria).
Comment: Room-temperature (RT) structure.
Entry added by: Ruyi Song Chemistry department, Duke university
Last updated on: Sept. 24, 2022, 9:02 p.m.
Last updated by: Harrison York Duke University
Download data
S-2-methylbutylammonium lead iodide: atomic structure
See all entries for this property (6 total)
Origin: experimental (T = 100.0 K)
Space group: P 2₁ 2₁ 2₁
Crystal system: orthorhombic
| a: | 29.28919983 Å |
| b: | 8.692399979 Å |
| c: | 8.701999664 Å |
| α: | 90° |
| β: | 90° |
| γ: | 90° |
- temperature = 100.0 K
Sample type: single crystal
Starting materials: S-2-MeBA, PbI2, HI, dimethylformamide (DMF), dichloromethane (DCM), ethyl ether
Product: yellow flaky single crystals (S-2-MeBA)2PbI4
Description: Add (S-2-MeBA) (0.25 mmol) and PbI2 (0.125 mmol) to 0.8 mL of an aqueous HI solution. Obtain a clear solution at 95 degrees Celsius, then cool to room temperature at a rate of 2 degrees Celsius per hour. The cooling process yields about 0.07 grams of (S-2-MeBA)2PbI4 crystals. Then, dissolve 0.03 g of the obtained crystals in dimethylformamide (DMF) (0.8 mol/L) in a small uncovered vial, and place it in a larger vial holding dichloromethane (DCM). Over a week-long period, the DCM vapors diffuse into the vial with the DMF solution and produce yellow flaky single crystals ((S-2-MeBA)2PbI4) as well as S-2-MeBA salt, light-yellow needle-shaped crystals. The crystals were filtered, washed with ethyl ether, and vacuum-dried. The (S-2-MeBA)2PbI4 crystals can be separated from the salt crystals under a microscope, and they were then used for single-crystal X-ray diffraction (SC-XRD).
Method: Single-crystal X-ray diffraction (SC-XRD)
Description: A Rigaku XtaLAB Synergy-S diffractometer using Mo Kα radiation (λ = 0.71073 Å) and functioning at 50 kV and 30 mA gathered data on (S-2-MeBA)2PbI4 crystals at room temperature (298 K). Data was also collected at 100 K, incorporating an 800 Series Cryostream Cooler. CrysAlisPro performed peak hunting, data reduction, and numerical absorption correction. SHELXS direct methods and SHELXL least-squares method solved crystal structures. PLATON’s ADDSYM tool analyzed the symmetry of full and isolated inorganic structures (default tolerance values and distance criteria).
Comment: 100K structure experienced slow-cooling process, low-temperature phase (LT).
Entry added by: Ruyi Song Chemistry department, Duke university
Last updated on: Sept. 24, 2022, 9:02 p.m.
Last updated by: Harrison York Duke University
Notice: This dataset has been transferred from https://materials.hybrid3.duke.edu/materials/485 . This was done in order to keep related structures of the same material together.
Download data
S-2-methylbutylammonium lead iodide: atomic structure
See all entries for this property (6 total)
Origin: experimental (T = 100.0 K)
Space group: P 2₁
Crystal system: monoclinic
| a: | 30.0807991 Å |
| b: | 8.692000389 Å |
| c: | 8.723230362 Å |
| α: | 90° |
| β: | 90° |
| γ: | 102.0960007° |
- temperature = 100.0 K
Sample type: single crystal
Starting materials: S-2-MeBA, PbI2, HI, dimethylformamide (DMF), dichloromethane (DCM), ethyl ether
Product: yellow flaky single crystals (S-2-MeBA)2PbI4
Description: Add (S-2-MeBA) (0.25 mmol) and PbI2 (0.125 mmol) to 0.8 mL of an aqueous HI solution. Obtain a clear solution at 95 degrees Celsius, then cool to room temperature at a rate of 2 degrees Celsius per hour. The cooling process yields about 0.07 grams of (S-2-MeBA)2PbI4 crystals. Then, dissolve 0.03 g of the obtained crystals in dimethylformamide (DMF) (0.8 mol/L) in a small uncovered vial, and place it in a larger vial holding dichloromethane (DCM). Over a week-long period, the DCM vapors diffuse into the vial with the DMF solution and produce yellow flaky single crystals ((S-2-MeBA)2PbI4) as well as S-2-MeBA salt, light-yellow needle-shaped crystals. The crystals were filtered, washed with ethyl ether, and vacuum-dried. The (S-2-MeBA)2PbI4 crystals can be separated from the salt crystals under a microscope, and they were then used for single-crystal X-ray diffraction (SC-XRD).
Method: Single-crystal X-ray diffraction (SC-XRD)
Description: A Rigaku XtaLAB Synergy-S diffractometer using Mo Kα radiation (λ = 0.71073 Å) and functioning at 50 kV and 30 mA gathered data on (S-2-MeBA)2PbI4 crystals at room temperature (298 K). Data was also collected at 100 K, incorporating an 800 Series Cryostream Cooler. CrysAlisPro performed peak hunting, data reduction, and numerical absorption correction. SHELXS direct methods and SHELXL least-squares method solved crystal structures. PLATON’s ADDSYM tool analyzed the symmetry of full and isolated inorganic structures (default tolerance values and distance criteria).
Comment: This is a special phase obtained by a fast cooling of the room temperature (RT) structure to 100K (quenching). Quenching: When (S-2-MeBA)2PbI4 crystals are cooled quickly through quenching, their structural transition is avoided. Quenching was performed by precooling the SC-XRD space to 100 K and directly applying the mounted crystal onto the goniometer head. The structure symmetry was then analyzed using PLATON, revealing that the full structure, isolated inorganic sublattice, and orientation of organic cations remained primarily unchanged. Differential scanning calorimetry (DSC): A TA Discovery DSC 2500 was used to carry out low-temperature conventional differential scanning calorimetry (DSC) measurements. The instrument was under helium purge with a 5 K/min ramp from 298 K to 124 K and back to 298 K (with use of an LN2P cooler). Sample powder was loaded into a Tzero Hermetic aluminum pan, with temperature and enthalpy calibrated in advance. A similar instrument was used to perform high-temperature DSC measurements under nitrogen purge. The ramp rate was 5 K/min from 298 K to 593 K. An aluminum pan was hermetically sealed to contain samples. The Flash DSC-1 instrument was used for ultrafast DSC measurements (Mettler Toledo). The UFS-1 chip-sensor was mounted and conditioned in the instrument, corrected in the range of 173 K to 723 K. (S-2-MeBA)2PbI4 crystals were placed on a glass slide, a single crystal flake isolated attached to the chip and set beneath an optical microscope. Silicon oil (type 47 V, 60,000) was applied to strengthen the thermal contact between the chip and crystal.
Entry added by: Ruyi Song Chemistry department, Duke university
Last updated on: Sept. 24, 2022, 7:10 p.m.
Last updated by: Harrison York Duke University
Notice: This dataset has been transferred from https://materials.hybrid3.duke.edu/materials/486 . This was done in order to keep related structures of the same material together.
Download data
S-2-methylbutylammonium lead bromide: atomic structure
See all entries for this property (2 total)
Origin: experimental (T = 100.0 K)
Space group: P 2₁
Crystal system: monoclinic
| a: | 15.31429958 Å |
| b: | 8.220509529 Å |
| c: | 8.172200203 Å |
| α: | 90° |
| β: | 100.7149963° |
| γ: | 90° |
- temperature = 100.0 K
Sample type: single crystal
Starting materials: S-2-MeBA, PbBr2, HI, dimethylformamide (DMF), dichloromethane (DCM), ethyl ether
Product: yellow flaky single crystals (S-2-MeBA)2PbBr4
Description: Add (S-2-MeBA) (0.25 mmol) and PbBr2 (0.125 mmol) to 0.8 mL of an aqueous HI solution. Obtain a clear solution at 95 degrees Celsius, then cool to room temperature at a rate of 2 degrees Celsius per hour. The cooling process yields about 0.07 grams of (S-2-MeBA)2PbBr4 crystals. Then, dissolve 0.03 g of the obtained crystals in dimethylformamide (DMF) (0.8 mol/L) in a small uncovered vial, and place it in a larger vial holding dichloromethane (DCM). Over a week-long period, the DCM vapors diffuse into the vial with the DMF solution and produce yellow flaky single crystals ((S-2-MeBA)2PbBr4) as well as S-2-MeBA salt, light-yellow needle-shaped crystals. The crystals were filtered, washed with ethyl ether, and vacuum-dried. The (S-2-MeBA)2PbBr4 crystals can be separated from the salt crystals under a microscope, and they were then used for single-crystal X-ray diffraction (SC-XRD).
Comment: Differential scanning calorimetry (DSC): A TA Discovery DSC 2500 was used to carry out low-temperature conventional differential scanning calorimetry (DSC) measurements. The instrument was under helium purge with a 5 K/min ramp from 298 K to 124 K and back to 298 K (with use of an LN2P cooler). Sample powder was loaded into a Tzero Hermetic aluminum pan, with temperature and enthalpy calibrated in advance. A similar instrument was used to perform high-temperature DSC measurements under nitrogen purge. The ramp rate was 5 K/min from 298 K to 603 K. An aluminum pan was hermetically sealed to contain samples.
Method: Differential scanning calorimetry (DSC)
Description: A TA Discovery DSC 2500 was used to carry out low-temperature conventional differential scanning calorimetry (DSC) measurements. The instrument was under helium purge with a 5 K/min ramp from 298 K to 124 K and back to 298 K (with use of an LN2P cooler). Sample powder was loaded into a Tzero Hermetic aluminum pan, with temperature and enthalpy calibrated in advance. A similar instrument was used to perform high-temperature DSC measurements under nitrogen purge. The ramp rate was 5 K/min from 298 K to 603 K. An aluminum pan was hermetically sealed to contain samples.
Entry added by: Ruyi Song Chemistry department, Duke university
Last updated on: Sept. 20, 2022, 10:52 a.m.
Last updated by: Harrison York Duke University
Download data
S-2-methylbutylammonium lead iodide (placeholder 485): atomic structure
See all entries for this property (2 total)
atomic structure (placeholder 485)
Origin: experimental
Crystal system: orthorhombic
| a: | 29.28919983 Å |
| b: | 8.692399979 Å |
| c: | 8.701999664 Å |
| α: | 90° |
| β: | 90° |
| γ: | 90° |
Sample type: single crystal
Starting materials: S-2-MeBA, PbI2, HI, dimethylformamide (DMF), dichloromethane (DCM), ethyl ether
Product: yellow flaky single crystals (S-2-MeBA)2PbI4
Description: Add (S-2-MeBA) (0.25 mmol) and PbI2 (0.125 mmol) to 0.8 mL of an aqueous HI solution. Obtain a clear solution at 95 degrees Celsius, then cool to room temperature at a rate of 2 degrees Celsius per hour. The cooling process yields about 0.07 grams of (S-2-MeBA)2PbI4 crystals. Then, dissolve 0.03 g of the obtained crystals in dimethylformamide (DMF) (0.8 mol/L) in a small uncovered vial, and place it in a larger vial holding dichloromethane (DCM). Over a week-long period, the DCM vapors diffuse into the vial with the DMF solution and produce yellow flaky single crystals ((S-2-MeBA)2PbI4) as well as S-2-MeBA salt, light-yellow needle-shaped crystals. The crystals were filtered, washed with ethyl ether, and vacuum-dried. The (S-2-MeBA)2PbI4 crystals can be separated from the salt crystals under a microscope, and they were then used for single-crystal X-ray diffraction (SC-XRD).
Method: Single-crystal X-ray diffraction (SC-XRD)
Description: A Rigaku XtaLAB Synergy-S diffractometer using Mo Kα radiation (λ = 0.71073 Å) and functioning at 50 kV and 30 mA gathered data on (S-2-MeBA)2PbI4 crystals at room temperature (298 K). Data was also collected at 100 K, incorporating an 800 Series Cryostream Cooler. CrysAlisPro performed peak hunting, data reduction, and numerical absorption correction. SHELXS direct methods and SHELXL least-squares method solved crystal structures. PLATON’s ADDSYM tool analyzed the symmetry of full and isolated inorganic structures (default tolerance values and distance criteria).
Entry added by: Harrison York Duke University
Last updated on: Sept. 24, 2022, 6:10 p.m.
Last updated by: Harrison York Duke University
Download data
S-2-methylbutylammonium lead iodide (placeholder 486): atomic structure
See all entries for this property (2 total)
atomic structure (placeholder 486)
Origin: experimental
Crystal system: monoclinic
| a: | 30.0807991 Å |
| b: | 8.692000389 Å |
| c: | 8.723230362 Å |
| α: | 90° |
| β: | 90° |
| γ: | 102.0960007° |
Sample type: single crystal
Starting materials: S-2-MeBA, PbI2, HI, dimethylformamide (DMF), dichloromethane (DCM), ethyl ether
Product: yellow flaky single crystals (S-2-MeBA)2PbI4
Description: Add (S-2-MeBA) (0.25 mmol) and PbI2 (0.125 mmol) to 0.8 mL of an aqueous HI solution. Obtain a clear solution at 95 degrees Celsius, then cool to room temperature at a rate of 2 degrees Celsius per hour. The cooling process yields about 0.07 grams of (S-2-MeBA)2PbI4 crystals. Then, dissolve 0.03 g of the obtained crystals in dimethylformamide (DMF) (0.8 mol/L) in a small uncovered vial, and place it in a larger vial holding dichloromethane (DCM). Over a week-long period, the DCM vapors diffuse into the vial with the DMF solution and produce yellow flaky single crystals ((S-2-MeBA)2PbI4) as well as S-2-MeBA salt, light-yellow needle-shaped crystals. The crystals were filtered, washed with ethyl ether, and vacuum-dried. The (S-2-MeBA)2PbI4 crystals can be separated from the salt crystals under a microscope, and they were then used for single-crystal X-ray diffraction (SC-XRD).
Method: Single-crystal X-ray diffraction (SC-XRD)
Description: A Rigaku XtaLAB Synergy-S diffractometer using Mo Kα radiation (λ = 0.71073 Å) and functioning at 50 kV and 30 mA gathered data on (S-2-MeBA)2PbI4 crystals at room temperature (298 K). Data was also collected at 100 K, incorporating an 800 Series Cryostream Cooler. CrysAlisPro performed peak hunting, data reduction, and numerical absorption correction. SHELXS direct methods and SHELXL least-squares method solved crystal structures. PLATON’s ADDSYM tool analyzed the symmetry of full and isolated inorganic structures (default tolerance values and distance criteria).
Comment: Quenching:When (S-2-MeBA)2PbI4 crystals are cooled quickly through quenching, their structural transition is avoided. Quenching was performed by precooling the SC-XRD space to 100 K and directly applying the mounted crystal onto the goniometer head. The structure symmetry was then analyzed using PLATON, revealing that the full structure, isolated inorganic sublattice, and orientation of organic cations remained primarily unchanged.Differential scanning calorimetry (DSC):A TA Discovery DSC 2500 was used to carry out low-temperature conventional differential scanning calorimetry (DSC) measurements. The instrument was under helium purge with a 5 K/min ramp from 298 K to 124 K and back to 298 K (with use of an LN2P cooler). Sample powder was loaded into a Tzero Hermetic aluminum pan, with temperature and enthalpy calibrated in advance.A similar instrument was used to perform high-temperature DSC measurements under nitrogen purge. The ramp rate was 5 K/min from 298 K to 593 K. An aluminum pan was hermetically sealed to contain samples.The Flash DSC-1 instrument was used for ultrafast DSC measurements (Mettler Toledo). The UFS-1 chip-sensor was mounted and conditioned in the instrument, corrected in the range of 173 K to 723 K. (S-2-MeBA)2PbI4 crystals were placed on a glass slide, a single crystal flake isolated attached to the chip and set beneath an optical microscope. Silicon oil (type 47 V, 60,000) was applied to strengthen the thermal contact between the chip and crystal.
Entry added by: Harrison York Duke University
Last updated on: Sept. 24, 2022, 6:14 p.m.
Last updated by: Harrison York Duke University
Download data
S-2-methylbutylammonium lead iodide: differential scanning calorimetry
Origin: experimental
Space group: P 2₁
Sample type: single crystal
Starting materials: S-2-MeBA, PbI2, HI, dimethylformamide (DMF), dichloromethane (DCM), ethyl ether
Product: yellow flaky single crystals (S-2-MeBA)2PbI4
Description: Add (S-2-MeBA) (0.25 mmol) and PbI2 (0.125 mmol) to 0.8 mL of an aqueous HI solution. Obtain a clear solution at 95 degrees Celsius, then cool to room temperature at a rate of 2 degrees Celsius per hour. The cooling process yields about 0.07 grams of (S-2-MeBA)2PbI4 crystals. Then, dissolve 0.03 g of the obtained crystals in dimethylformamide (DMF) (0.8 mol/L) in a small uncovered vial, and place it in a larger vial holding dichloromethane (DCM). Over a week-long period, the DCM vapors diffuse into the vial with the DMF solution and produce yellow flaky single crystals ((S-2-MeBA)2PbI4) as well as S-2-MeBA salt, light-yellow needle-shaped crystals. The crystals were filtered, washed with ethyl ether, and vacuum-dried. The (S-2-MeBA)2PbI4 crystals can be separated from the salt crystals under a microscope, and they were then used for single-crystal X-ray diffraction (SC-XRD).
Method: Differential scanning calorimetry (DSC)
Description: A TA Discovery DSC 2500 was used to carry out low-temperature conventional differential scanning calorimetry (DSC) measurements. The instrument was under helium purge with a 5 K/min ramp from 298 K to 124 K and back to 298 K (with use of an LN2P cooler). Sample powder was loaded into a Tzero Hermetic aluminum pan, with temperature and enthalpy calibrated in advance. A similar instrument was used to perform high-temperature DSC measurements under nitrogen purge. The ramp rate was 5 K/min from 298 K to 603 K. An aluminum pan was hermetically sealed to contain samples. Graph: Cooling (5 K/min) of crystal with slow process is the upper graph. Notable points at 183 K and 188 K, where the structure transitions. Heating (5 K/min) of crystal with slow process is the lower graph. Notable points at 203 K and 213 K, where the structure transitions.
Entry added on: Sept. 24, 2022, 8:54 p.m.
Entry added by: Harrison York Duke University
Last updated on: Feb. 22, 2023, 9:42 p.m.
Last updated by: Harrison York Duke University
Download data
S-2-methylbutylammonium lead iodide: band structure
See all entries for this property (3 total)
T = 298.0 K
Origin: experimental
Space group: P 2₁
Crystal system: monoclinic
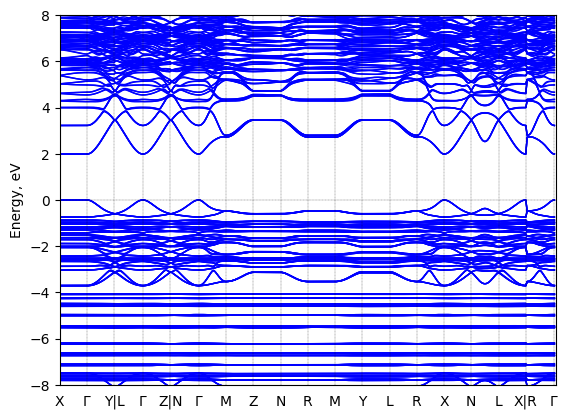
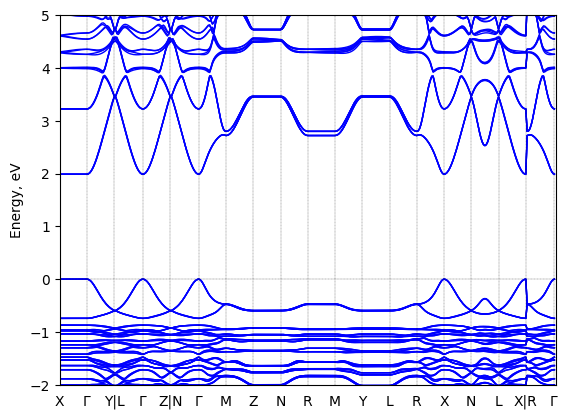
Sample type: single crystal
Starting materials: S-2-MeBA, PbI2, HI, dimethylformamide (DMF), dichloromethane (DCM), ethyl ether
Product: yellow flaky single crystals (S-2-MeBA)2PbI4
Description: Add (S-2-MeBA) (0.25 mmol) and PbI2 (0.125 mmol) to 0.8 mL of an aqueous HI solution. Obtain a clear solution at 95 degrees Celsius, then cool to room temperature at a rate of 2 degrees Celsius per hour. The cooling process yields about 0.07 grams of (S-2-MeBA)2PbI4 crystals. Then, dissolve 0.03 g of the obtained crystals in dimethylformamide (DMF) (0.8 mol/L) in a small uncovered vial, and place it in a larger vial holding dichloromethane (DCM). Over a week-long period, the DCM vapors diffuse into the vial with the DMF solution and produce yellow flaky single crystals ((S-2-MeBA)2PbI4) as well as S-2-MeBA salt, light-yellow needle-shaped crystals. The crystals were filtered, washed with ethyl ether, and vacuum-dried. The (S-2-MeBA)2PbI4 crystals can be separated from the salt crystals under a microscope, and they were then used for single-crystal X-ray diffraction (SC-XRD).
Code: FHI-aims
K-point grid: 3x4x4
Level of relativity: relativistic atomic ZORA scalar, include spin orbit
Entry added on: Oct. 3, 2022, 10:47 p.m.
Entry added by: Harrison York Duke University
Last updated on: Jan. 17, 2023, 2:37 p.m.
Last updated by: Harrison York Duke University
Download data
S-2-methylbutylammonium lead iodide: band structure
See all entries for this property (3 total)
T = 100 K LT
Origin: experimental
Space group: P 2₁ 2₁ 2₁
Crystal system: monoclinic
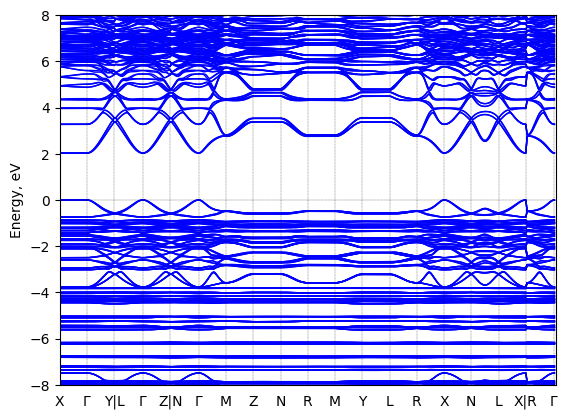
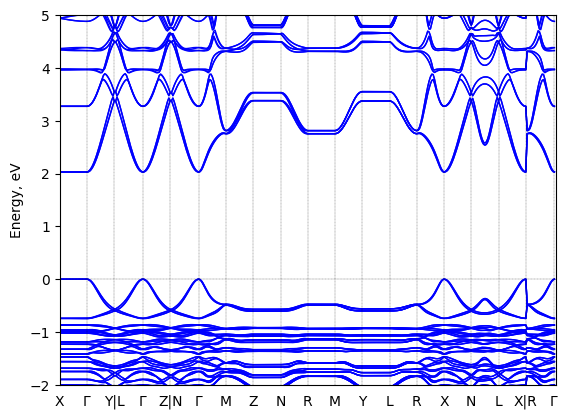
Sample type: single crystal
Starting materials: S-2-MeBA, PbI2, HI, dimethylformamide (DMF), dichloromethane (DCM), ethyl ether
Product: yellow flaky single crystals (S-2-MeBA)2PbI4
Description: Add (S-2-MeBA) (0.25 mmol) and PbI2 (0.125 mmol) to 0.8 mL of an aqueous HI solution. Obtain a clear solution at 95 degrees Celsius, then cool to room temperature at a rate of 2 degrees Celsius per hour. The cooling process yields about 0.07 grams of (S-2-MeBA)2PbI4 crystals. Then, dissolve 0.03 g of the obtained crystals in dimethylformamide (DMF) (0.8 mol/L) in a small uncovered vial, and place it in a larger vial holding dichloromethane (DCM). Over a week-long period, the DCM vapors diffuse into the vial with the DMF solution and produce yellow flaky single crystals ((S-2-MeBA)2PbI4) as well as S-2-MeBA salt, light-yellow needle-shaped crystals. The crystals were filtered, washed with ethyl ether, and vacuum-dried. The (S-2-MeBA)2PbI4 crystals can be separated from the salt crystals under a microscope, and they were then used for single-crystal X-ray diffraction (SC-XRD).
Code: FHI-aims
K-point grid: 3x4x4
Basis set definition: relativistic atomic ZORA scalar, include spin orbit
Entry added on: Oct. 3, 2022, 10:50 p.m.
Entry added by: Harrison York Duke University
Last updated on: Oct. 4, 2022, 10:28 a.m.
Last updated by: Harrison York Duke University
Download data
S-2-methylbutylammonium lead iodide: band structure
See all entries for this property (3 total)
T = 100 K quenched
Origin: experimental
Space group: P 2₁
Crystal system: monoclinic
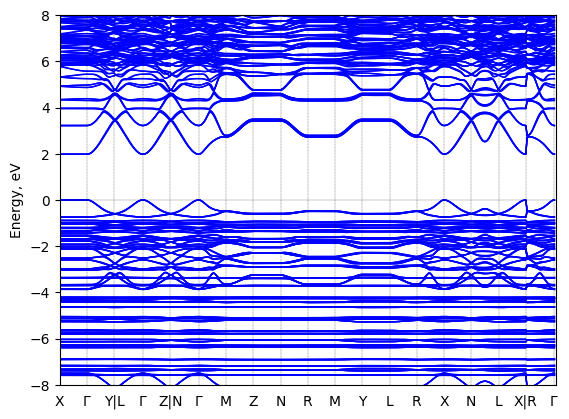
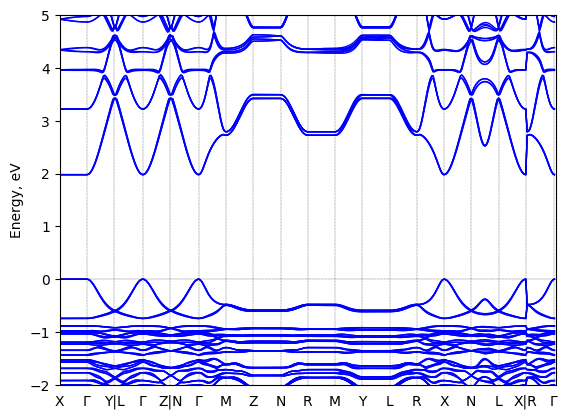
Sample type: single crystal
Starting materials: S-2-MeBA, PbI2, HI, dimethylformamide (DMF), dichloromethane (DCM), ethyl ether
Product: yellow flaky single crystals (S-2-MeBA)2PbI4
Description: Add (S-2-MeBA) (0.25 mmol) and PbI2 (0.125 mmol) to 0.8 mL of an aqueous HI solution. Obtain a clear solution at 95 degrees Celsius, then cool to room temperature at a rate of 2 degrees Celsius per hour. The cooling process yields about 0.07 grams of (S-2-MeBA)2PbI4 crystals. Then, dissolve 0.03 g of the obtained crystals in dimethylformamide (DMF) (0.8 mol/L) in a small uncovered vial, and place it in a larger vial holding dichloromethane (DCM). Over a week-long period, the DCM vapors diffuse into the vial with the DMF solution and produce yellow flaky single crystals ((S-2-MeBA)2PbI4) as well as S-2-MeBA salt, light-yellow needle-shaped crystals. The crystals were filtered, washed with ethyl ether, and vacuum-dried. The (S-2-MeBA)2PbI4 crystals can be separated from the salt crystals under a microscope, and they were then used for single-crystal X-ray diffraction (SC-XRD).
K-point grid: 3x4x4
Level of relativity: relativistic atomic ZORA scalar, include spin orbit
Entry added on: Oct. 3, 2022, 10:56 p.m.
Entry added by: Harrison York Duke University
Last updated on: Oct. 4, 2022, 10:29 a.m.
Last updated by: Harrison York Duke University
Download data
S-2-methylbutylammonium lead bromide: band structure
See all entries for this property (2 total)
T = 100 K
Origin: experimental
Space group: P 2₁ 2₁ 2₁
Crystal system: monoclinic
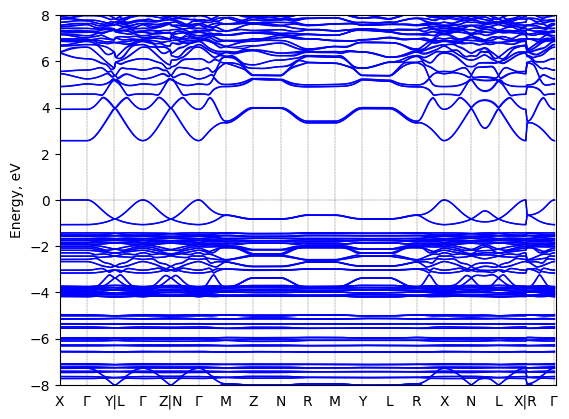
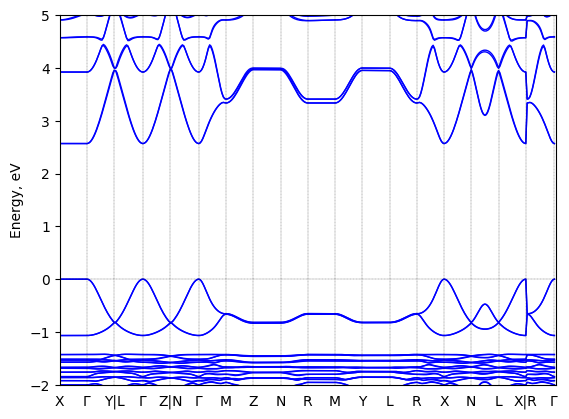
Sample type: single crystal
Starting materials: S-2-MeBA, PbBr2, HI, dimethylformamide (DMF), dichloromethane (DCM), ethyl ether
Product: yellow flaky single crystals (S-2-MeBA)2PbBr4
Description: Add (S-2-MeBA) (0.25 mmol) and PbBr2 (0.125 mmol) to 0.8 mL of an aqueous HI solution. Obtain a clear solution at 95 degrees Celsius, then cool to room temperature at a rate of 2 degrees Celsius per hour. The cooling process yields about 0.07 grams of (S-2-MeBA)2PbBr4 crystals. Then, dissolve 0.03 g of the obtained crystals in dimethylformamide (DMF) (0.8 mol/L) in a small uncovered vial, and place it in a larger vial holding dichloromethane (DCM). Over a week-long period, the DCM vapors diffuse into the vial with the DMF solution and produce yellow flaky single crystals ((S-2-MeBA)2PbBr4) as well as S-2-MeBA salt, light-yellow needle-shaped crystals. The crystals were filtered, washed with ethyl ether, and vacuum-dried. The (S-2-MeBA)2PbBr4 crystals can be separated from the salt crystals under a microscope, and they were then used for single-crystal X-ray diffraction (SC-XRD).
Code: FHI-aims
Level of theory: density functional theory
Exchange-correlation functional: HSE06 [α=0.25, ω=0.11 Å^(-1)]
K-point grid: 3x4x4
Level of relativity: relativistic atomic ZORA scalar, include spin orbit
Basis set definition: FHI-aims intermediate settings
Numerical accuracy: FHI-aims intermediate settings
Entry added on: Oct. 3, 2022, 11:02 p.m.
Entry added by: Harrison York Duke University
Last updated on: Oct. 6, 2022, 5:44 p.m.
Last updated by: Harrison York Duke University
Download data
S-2-methylbutylammonium lead bromide: band structure
See all entries for this property (2 total)
T = 298.0 K
Origin: experimental
Space group: P 2₁
Crystal system: monoclinic
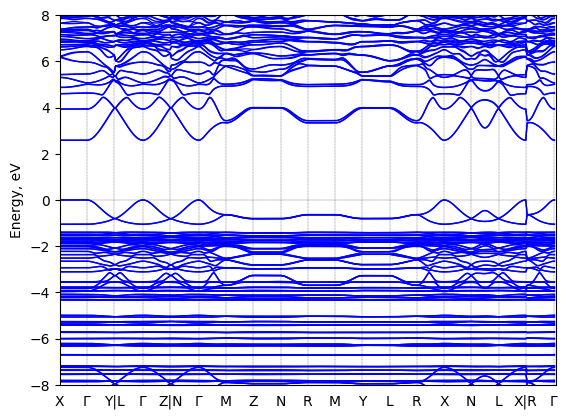
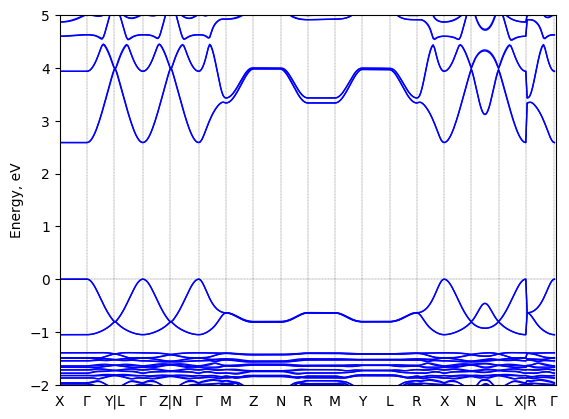
Sample type: single crystal
Starting materials: S-2-MeBA, PbBr2, HI, dimethylformamide (DMF), dichloromethane (DCM), ethyl ether
Product: yellow flaky single crystals (S-2-MeBA)2PbBr4
Description: Add (S-2-MeBA) (0.25 mmol) and PbBr2 (0.125 mmol) to 0.8 mL of an aqueous HI solution. Obtain a clear solution at 95 degrees Celsius, then cool to room temperature at a rate of 2 degrees Celsius per hour. The cooling process yields about 0.07 grams of (S-2-MeBA)2PbBr4 crystals. Then, dissolve 0.03 g of the obtained crystals in dimethylformamide (DMF) (0.8 mol/L) in a small uncovered vial, and place it in a larger vial holding dichloromethane (DCM). Over a week-long period, the DCM vapors diffuse into the vial with the DMF solution and produce yellow flaky single crystals ((S-2-MeBA)2PbBr4) as well as S-2-MeBA salt, light-yellow needle-shaped crystals. The crystals were filtered, washed with ethyl ether, and vacuum-dried. The (S-2-MeBA)2PbBr4 crystals can be separated from the salt crystals under a microscope, and they were then used for single-crystal X-ray diffraction (SC-XRD).
Code: FHI-aims
Level of theory: density functional theory
Exchange-correlation functional: HSE06 [α=0.25, ω=0.11 Å^(-1)]
K-point grid: 3x4x4
Level of relativity: relativistic atomic ZORA scalar, include_spin_orbit
Basis set definition: FHI-aims intermediate settings
Numerical accuracy: FHI-aims intermediate settings
Entry added on: Oct. 3, 2022, 11:18 p.m.
Entry added by: Harrison York Duke University
Last updated on: Oct. 6, 2022, 6:36 p.m.
Last updated by: Harrison York Duke University
Download data
Tributyl(methyl)phosphonium lead iodide: band structure
T = 298.0 K
Origin: experimental
Crystal system: triclinic
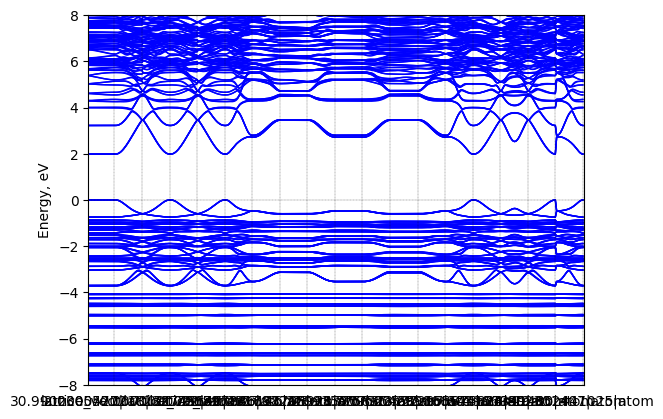
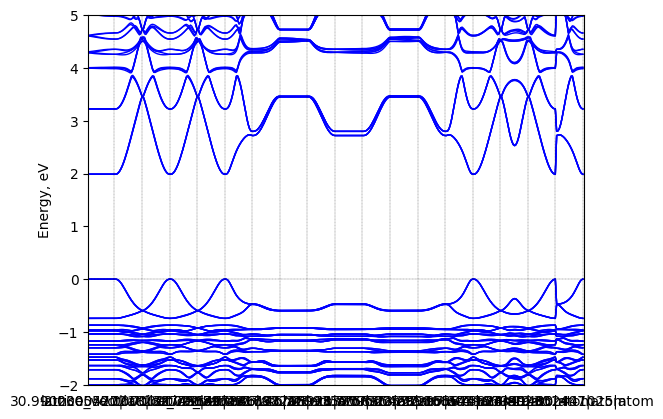
Sample type: single crystal
Starting materials: S-2-MeBA, PbI2, HI, dimethylformamide (DMF), dichloromethane (DCM), ethyl ether
Product: yellow flaky single crystals (S-2-MeBA)2PbI4
Description: Add (S-2-MeBA) (0.25 mmol) and PbI2 (0.125 mmol) to 0.8 mL of an aqueous HI solution. Obtain a clear solution at 95 degrees Celsius, then cool to room temperature at a rate of 2 degrees Celsius per hour. The cooling process yields about 0.07 grams of (S-2-MeBA)2PbI4 crystals. Then, dissolve 0.03 g of the obtained crystals in dimethylformamide (DMF) (0.8 mol/L) in a small uncovered vial, and place it in a larger vial holding dichloromethane (DCM). Over a week-long period, the DCM vapors diffuse into the vial with the DMF solution and produce yellow flaky single crystals ((S-2-MeBA)2PbI4) as well as S-2-MeBA salt, light-yellow needle-shaped crystals. The crystals were filtered, washed with ethyl ether, and vacuum-dried. The (S-2-MeBA)2PbI4 crystals can be separated from the salt crystals under a microscope, and they were then used for single-crystal X-ray diffraction (SC-XRD).
Code: FHI-aims
K-point grid: 3x4x4
Level of relativity: relativistic atomic ZORA scalar, include spin orbit
Entry added on: Jan. 17, 2023, 3 p.m.
Entry added by: Harrison York Duke University
Last updated on: Jan. 17, 2023, 3 p.m.
Last updated by: Harrison York Duke University
Download data
S-2-methylbutylammonium lead iodide: atomic structure
See all entries for this property (6 total)
H_relaxed_structure
Origin: computational
Space group: P 2₁
Crystal system: monoclinic
| a: | 30.99020004 Å |
| b: | 8.777999877 Å |
| c: | 8.8149004 Å |
| α: | 90° |
| β: | 90° |
| γ: | 103.375° |
Sample type: single crystal
Starting materials: S-2-MeBA, PbI2, HI, dimethylformamide (DMF), dichloromethane (DCM), ethyl ether
Product: yellow flaky single crystals (S-2-MeBA)2PbI4
Description: Add (S-2-MeBA) (0.25 mmol) and PbI2 (0.125 mmol) to 0.8 mL of an aqueous HI solution. Obtain a clear solution at 95 degrees Celsius, then cool to room temperature at a rate of 2 degrees Celsius per hour. The cooling process yields about 0.07 grams of (S-2-MeBA)2PbI4 crystals. Then, dissolve 0.03 g of the obtained crystals in dimethylformamide (DMF) (0.8 mol/L) in a small uncovered vial, and place it in a larger vial holding dichloromethane (DCM). Over a week-long period, the DCM vapors diffuse into the vial with the DMF solution and produce yellow flaky single crystals ((S-2-MeBA)2PbI4) as well as S-2-MeBA salt, light-yellow needle-shaped crystals. The crystals were filtered, washed with ethyl ether, and vacuum-dried. The (S-2-MeBA)2PbI4 crystals can be separated from the salt crystals under a microscope, and they were then used for single-crystal X-ray diffraction (SC-XRD).
Method: Single-crystal X-ray diffraction (SC-XRD)
Description: A Rigaku XtaLAB Synergy-S diffractometer using Mo Kα radiation (λ = 0.71073 Å) and functioning at 50 kV and 30 mA gathered data on (S-2-MeBA)2PbI4 crystals at room temperature (298 K). Data was also collected at 100 K, incorporating an 800 Series Cryostream Cooler. CrysAlisPro performed peak hunting, data reduction, and numerical absorption correction. SHELXS direct methods and SHELXL least-squares method solved crystal structures. PLATON’s ADDSYM tool analyzed the symmetry of full and isolated inorganic structures (default tolerance values and distance criteria).
Comment: Room-temperature (RT) structure.
Code: FHI-aims
Level of theory: Density Functional Theory (DFT)
Exchange-correlation functional: PBE-TS
K-point grid: 2X5X5
Level of relativity: atomic ZORA
Basis set definition: intermediate
Entry added by: Chunyu Chen Mat. Sci Duke University
Last updated on: May 8, 2025, 1:24 p.m.
Last updated by: Chunyu Chen Mat. Sci Duke University
Download data
S-2-methylbutylammonium lead iodide: atomic structure
See all entries for this property (6 total)
H_relaxed_structure
Origin: computational
Space group: P 2₁ 2₁ 2₁
Crystal system: orthorhombic
| a: | 29.28919983 Å |
| b: | 8.69239998 Å |
| c: | 8.70199966 Å |
| α: | 90° |
| β: | 90° |
| γ: | 90° |
Sample type: single crystal
Starting materials: S-2-MeBA, PbI2, HI, dimethylformamide (DMF), dichloromethane (DCM), ethyl ether
Product: yellow flaky single crystals (S-2-MeBA)2PbI4
Description: Add (S-2-MeBA) (0.25 mmol) and PbI2 (0.125 mmol) to 0.8 mL of an aqueous HI solution. Obtain a clear solution at 95 degrees Celsius, then cool to room temperature at a rate of 2 degrees Celsius per hour. The cooling process yields about 0.07 grams of (S-2-MeBA)2PbI4 crystals. Then, dissolve 0.03 g of the obtained crystals in dimethylformamide (DMF) (0.8 mol/L) in a small uncovered vial, and place it in a larger vial holding dichloromethane (DCM). Over a week-long period, the DCM vapors diffuse into the vial with the DMF solution and produce yellow flaky single crystals ((S-2-MeBA)2PbI4) as well as S-2-MeBA salt, light-yellow needle-shaped crystals. The crystals were filtered, washed with ethyl ether, and vacuum-dried. The (S-2-MeBA)2PbI4 crystals can be separated from the salt crystals under a microscope, and they were then used for single-crystal X-ray diffraction (SC-XRD).
Method: Single-crystal X-ray diffraction (SC-XRD)
Description: A Rigaku XtaLAB Synergy-S diffractometer using Mo Kα radiation (λ = 0.71073 Å) and functioning at 50 kV and 30 mA gathered data on (S-2-MeBA)2PbI4 crystals at room temperature (298 K). Data was also collected at 100 K, incorporating an 800 Series Cryostream Cooler. CrysAlisPro performed peak hunting, data reduction, and numerical absorption correction. SHELXS direct methods and SHELXL least-squares method solved crystal structures. PLATON’s ADDSYM tool analyzed the symmetry of full and isolated inorganic structures (default tolerance values and distance criteria).
Comment: 100K structure experienced slow-cooling process, low-temperature phase (LT).
Code: FHI-aims
Level of theory: Density Functional Theory (DFT)
Exchange-correlation functional: PBE-TS
K-point grid: 2X5X5
Level of relativity: atomic ZORA
Basis set definition: intermediate
Entry added by: Chunyu Chen Mat. Sci Duke University
Last updated on: May 8, 2025, 1:26 p.m.
Last updated by: Chunyu Chen Mat. Sci Duke University
Download data
S-2-methylbutylammonium lead iodide: atomic structure
See all entries for this property (6 total)
H_relaxed_structure
Origin: computational
Space group: P 2₁
Crystal system: monoclinic
| a: | 30.0807991 Å |
| b: | 8.692000387 Å |
| c: | 8.72323036 Å |
| α: | 90° |
| β: | 90° |
| γ: | 102.0960007° |
Sample type: single crystal
Starting materials: S-2-MeBA, PbI2, HI, dimethylformamide (DMF), dichloromethane (DCM), ethyl ether
Product: yellow flaky single crystals (S-2-MeBA)2PbI4
Description: Add (S-2-MeBA) (0.25 mmol) and PbI2 (0.125 mmol) to 0.8 mL of an aqueous HI solution. Obtain a clear solution at 95 degrees Celsius, then cool to room temperature at a rate of 2 degrees Celsius per hour. The cooling process yields about 0.07 grams of (S-2-MeBA)2PbI4 crystals. Then, dissolve 0.03 g of the obtained crystals in dimethylformamide (DMF) (0.8 mol/L) in a small uncovered vial, and place it in a larger vial holding dichloromethane (DCM). Over a week-long period, the DCM vapors diffuse into the vial with the DMF solution and produce yellow flaky single crystals ((S-2-MeBA)2PbI4) as well as S-2-MeBA salt, light-yellow needle-shaped crystals. The crystals were filtered, washed with ethyl ether, and vacuum-dried. The (S-2-MeBA)2PbI4 crystals can be separated from the salt crystals under a microscope, and they were then used for single-crystal X-ray diffraction (SC-XRD).
Method: Single-crystal X-ray diffraction (SC-XRD)
Description: A Rigaku XtaLAB Synergy-S diffractometer using Mo Kα radiation (λ = 0.71073 Å) and functioning at 50 kV and 30 mA gathered data on (S-2-MeBA)2PbI4 crystals at room temperature (298 K). Data was also collected at 100 K, incorporating an 800 Series Cryostream Cooler. CrysAlisPro performed peak hunting, data reduction, and numerical absorption correction. SHELXS direct methods and SHELXL least-squares method solved crystal structures. PLATON’s ADDSYM tool analyzed the symmetry of full and isolated inorganic structures (default tolerance values and distance criteria).
Comment: This is a special phase obtained by a fast cooling of the room temperature (RT) structure to 100K (quenching).Quenching:When (S-2-MeBA)2PbI4 crystals are cooled quickly through quenching, their structural transition is avoided. Quenching was performed by precooling the SC-XRD space to 100 K and directly applying the mounted crystal onto the goniometer head. The structure symmetry was then analyzed using PLATON, revealing that the full structure, isolated inorganic sublattice, and orientation of organic cations remained primarily unchanged.Differential scanning calorimetry (DSC):A TA Discovery DSC 2500 was used to carry out low-temperature conventional differential scanning calorimetry (DSC) measurements. The instrument was under helium purge with a 5 K/min ramp from 298 K to 124 K and back to 298 K (with use of an LN2P cooler). Sample powder was loaded into a Tzero Hermetic aluminum pan, with temperature and enthalpy calibrated in advance.A similar instrument was used to perform high-temperature DSC measurements under nitrogen purge. The ramp rate was 5 K/min from 298 K to 593 K. An aluminum pan was hermetically sealed to contain samples.The Flash DSC-1 instrument was used for ultrafast DSC measurements (Mettler Toledo). The UFS-1 chip-sensor was mounted and conditioned in the instrument, corrected in the range of 173 K to 723 K. (S-2-MeBA)2PbI4 crystals were placed on a glass slide, a single crystal flake isolated attached to the chip and set beneath an optical microscope. Silicon oil (type 47 V, 60,000) was applied to strengthen the thermal contact between the chip and crystal.
Code: FHI-aims
Level of theory: Density Functional Theory (DFT)
Exchange-correlation functional: PBE-TS
K-point grid: 2X5X5
Level of relativity: atomic ZORA
Basis set definition: intermediate
Entry added by: Chunyu Chen Mat. Sci Duke University
Last updated on: May 8, 2025, 1:33 p.m.
Last updated by: Chunyu Chen Mat. Sci Duke University
Download data
S-2-methylbutylammonium lead bromide: atomic structure
See all entries for this property (2 total)
H_relaxed_structure
Origin: computational
Space group: P 2₁
Crystal system: monoclinic
| a: | 15.31429958 Å |
| b: | 8.22050953 Å |
| c: | 8.172200204 Å |
| α: | 90° |
| β: | 100.7149963° |
| γ: | 90° |
Sample type: single crystal
Starting materials: S-2-MeBA, PbBr2, HI, dimethylformamide (DMF), dichloromethane (DCM), ethyl ether
Product: yellow flaky single crystals (S-2-MeBA)2PbBr4
Description: Add (S-2-MeBA) (0.25 mmol) and PbBr2 (0.125 mmol) to 0.8 mL of an aqueous HI solution. Obtain a clear solution at 95 degrees Celsius, then cool to room temperature at a rate of 2 degrees Celsius per hour. The cooling process yields about 0.07 grams of (S-2-MeBA)2PbBr4 crystals. Then, dissolve 0.03 g of the obtained crystals in dimethylformamide (DMF) (0.8 mol/L) in a small uncovered vial, and place it in a larger vial holding dichloromethane (DCM). Over a week-long period, the DCM vapors diffuse into the vial with the DMF solution and produce yellow flaky single crystals ((S-2-MeBA)2PbBr4) as well as S-2-MeBA salt, light-yellow needle-shaped crystals. The crystals were filtered, washed with ethyl ether, and vacuum-dried. The (S-2-MeBA)2PbBr4 crystals can be separated from the salt crystals under a microscope, and they were then used for single-crystal X-ray diffraction (SC-XRD).
Comment: Differential scanning calorimetry (DSC):A TA Discovery DSC 2500 was used to carry out low-temperature conventional differential scanning calorimetry (DSC) measurements. The instrument was under helium purge with a 5 K/min ramp from 298 K to 124 K and back to 298 K (with use of an LN2P cooler). Sample powder was loaded into a Tzero Hermetic aluminum pan, with temperature and enthalpy calibrated in advance.A similar instrument was used to perform high-temperature DSC measurements under nitrogen purge. The ramp rate was 5 K/min from 298 K to 603 K. An aluminum pan was hermetically sealed to contain samples.
Method: Differential scanning calorimetry (DSC)
Description: A TA Discovery DSC 2500 was used to carry out low-temperature conventional differential scanning calorimetry (DSC) measurements. The instrument was under helium purge with a 5 K/min ramp from 298 K to 124 K and back to 298 K (with use of an LN2P cooler). Sample powder was loaded into a Tzero Hermetic aluminum pan, with temperature and enthalpy calibrated in advance. A similar instrument was used to perform high-temperature DSC measurements under nitrogen purge. The ramp rate was 5 K/min from 298 K to 603 K. An aluminum pan was hermetically sealed to contain samples.
Code: FHI-aims
Level of theory: Density Functional Theory (DFT)
Exchange-correlation functional: PBE-TS
K-point grid: 3X5X5
Level of relativity: atomic ZORA
Basis set definition: intermediate
Entry added by: Chunyu Chen Mat. Sci Duke University
Last updated on: May 8, 2025, 1:36 p.m.
Last updated by: Chunyu Chen Mat. Sci Duke University
Download data
S-2-methylbutylammonium lead iodide (placeholder 485): atomic structure
See all entries for this property (2 total)
H_relaxed_structure
Origin: computational
Crystal system: orthorhombic
| a: | 29.28919983 Å |
| b: | 8.69239998 Å |
| c: | 8.70199966 Å |
| α: | 90° |
| β: | 90° |
| γ: | 90° |
Sample type: single crystal
Starting materials: S-2-MeBA, PbI2, HI, dimethylformamide (DMF), dichloromethane (DCM), ethyl ether
Product: yellow flaky single crystals (S-2-MeBA)2PbI4
Description: Add (S-2-MeBA) (0.25 mmol) and PbI2 (0.125 mmol) to 0.8 mL of an aqueous HI solution. Obtain a clear solution at 95 degrees Celsius, then cool to room temperature at a rate of 2 degrees Celsius per hour. The cooling process yields about 0.07 grams of (S-2-MeBA)2PbI4 crystals. Then, dissolve 0.03 g of the obtained crystals in dimethylformamide (DMF) (0.8 mol/L) in a small uncovered vial, and place it in a larger vial holding dichloromethane (DCM). Over a week-long period, the DCM vapors diffuse into the vial with the DMF solution and produce yellow flaky single crystals ((S-2-MeBA)2PbI4) as well as S-2-MeBA salt, light-yellow needle-shaped crystals. The crystals were filtered, washed with ethyl ether, and vacuum-dried. The (S-2-MeBA)2PbI4 crystals can be separated from the salt crystals under a microscope, and they were then used for single-crystal X-ray diffraction (SC-XRD).
Method: Single-crystal X-ray diffraction (SC-XRD)
Description: A Rigaku XtaLAB Synergy-S diffractometer using Mo Kα radiation (λ = 0.71073 Å) and functioning at 50 kV and 30 mA gathered data on (S-2-MeBA)2PbI4 crystals at room temperature (298 K). Data was also collected at 100 K, incorporating an 800 Series Cryostream Cooler. CrysAlisPro performed peak hunting, data reduction, and numerical absorption correction. SHELXS direct methods and SHELXL least-squares method solved crystal structures. PLATON’s ADDSYM tool analyzed the symmetry of full and isolated inorganic structures (default tolerance values and distance criteria).
Code: FHI-aims
Level of theory: Density Functional Theory (DFT)
Exchange-correlation functional: PBE-TS
K-point grid: 2X5X5
Level of relativity: atomic ZORA
Basis set definition: intermediate
Entry added by: Chunyu Chen Mat. Sci Duke University
Last updated on: May 8, 2025, 2:37 p.m.
Last updated by: Chunyu Chen Mat. Sci Duke University
Download data
S-2-methylbutylammonium lead iodide (placeholder 486): atomic structure
See all entries for this property (2 total)
H_relaxed_structure
Origin: experimental
Crystal system: monoclinic
| a: | 30.0807991 Å |
| b: | 8.692000387 Å |
| c: | 8.72323036 Å |
| α: | 90° |
| β: | 90° |
| γ: | 102.0960007° |
Sample type: single crystal
Starting materials: S-2-MeBA, PbI2, HI, dimethylformamide (DMF), dichloromethane (DCM), ethyl ether
Product: yellow flaky single crystals (S-2-MeBA)2PbI4
Description: Add (S-2-MeBA) (0.25 mmol) and PbI2 (0.125 mmol) to 0.8 mL of an aqueous HI solution. Obtain a clear solution at 95 degrees Celsius, then cool to room temperature at a rate of 2 degrees Celsius per hour. The cooling process yields about 0.07 grams of (S-2-MeBA)2PbI4 crystals. Then, dissolve 0.03 g of the obtained crystals in dimethylformamide (DMF) (0.8 mol/L) in a small uncovered vial, and place it in a larger vial holding dichloromethane (DCM). Over a week-long period, the DCM vapors diffuse into the vial with the DMF solution and produce yellow flaky single crystals ((S-2-MeBA)2PbI4) as well as S-2-MeBA salt, light-yellow needle-shaped crystals. The crystals were filtered, washed with ethyl ether, and vacuum-dried. The (S-2-MeBA)2PbI4 crystals can be separated from the salt crystals under a microscope, and they were then used for single-crystal X-ray diffraction (SC-XRD).
Method: Single-crystal X-ray diffraction (SC-XRD)
Description: A Rigaku XtaLAB Synergy-S diffractometer using Mo Kα radiation (λ = 0.71073 Å) and functioning at 50 kV and 30 mA gathered data on (S-2-MeBA)2PbI4 crystals at room temperature (298 K). Data was also collected at 100 K, incorporating an 800 Series Cryostream Cooler. CrysAlisPro performed peak hunting, data reduction, and numerical absorption correction. SHELXS direct methods and SHELXL least-squares method solved crystal structures. PLATON’s ADDSYM tool analyzed the symmetry of full and isolated inorganic structures (default tolerance values and distance criteria).
Comment: Quenching:When (S-2-MeBA)2PbI4 crystals are cooled quickly through quenching, their structural transition is avoided. Quenching was performed by precooling the SC-XRD space to 100 K and directly applying the mounted crystal onto the goniometer head. The structure symmetry was then analyzed using PLATON, revealing that the full structure, isolated inorganic sublattice, and orientation of organic cations remained primarily unchanged.Differential scanning calorimetry (DSC):A TA Discovery DSC 2500 was used to carry out low-temperature conventional differential scanning calorimetry (DSC) measurements. The instrument was under helium purge with a 5 K/min ramp from 298 K to 124 K and back to 298 K (with use of an LN2P cooler). Sample powder was loaded into a Tzero Hermetic aluminum pan, with temperature and enthalpy calibrated in advance.A similar instrument was used to perform high-temperature DSC measurements under nitrogen purge. The ramp rate was 5 K/min from 298 K to 593 K. An aluminum pan was hermetically sealed to contain samples.The Flash DSC-1 instrument was used for ultrafast DSC measurements (Mettler Toledo). The UFS-1 chip-sensor was mounted and conditioned in the instrument, corrected in the range of 173 K to 723 K. (S-2-MeBA)2PbI4 crystals were placed on a glass slide, a single crystal flake isolated attached to the chip and set beneath an optical microscope. Silicon oil (type 47 V, 60,000) was applied to strengthen the thermal contact between the chip and crystal.
Code: FHI-aims
Level of theory: Density Functional Theory (DFT)
Exchange-correlation functional: PBE-TS
K-point grid: 2X5X5
Level of relativity: atomic ZORA
Basis set definition: intermediate
Entry added by: Chunyu Chen Mat. Sci Duke University
Last updated on: May 8, 2025, 2:38 p.m.
Last updated by: Chunyu Chen Mat. Sci Duke University
Download data