5,5‘‘‘-bis(aminoethyl)-2,2‘:5‘,2‘‘:5‘‘,2‘‘‘-quaterthiophene lead chloride
Chemical Formula: C20H22N2S4PbCl4
IUPAC: 5,5'''-bis(aminoethyl)-2,2':5',2'':5'',2'''-quaterthiophene lead(II) chloride
Alternate Names: 5,5'''-bis(aminoethyl)-2,2':5',2'':5'',2'''-quaterthiophene tetrachloroplumbate(II), AE4TPbCl4, (AEQT)PbCl4, AEQTPbCl4, C20H22S4N2PbCl4
Organic: C20H22N2S4
Inorganic: PbCl4, Lead chloride
Dimensionality: 2D n: 1
Formal Stoichiometry:
C : 20
,
H : 22
,
N : 2
,
S : 4
,
Pb : 1
,
Cl : 4
Atomic structure Verified
See all entries for this property (2 total)
Origin: computational
Crystal system: triclinic
| a: | 40.85056467 Å |
| b: | 11.29489724 Å |
| c: | 10.94887555 Å |
| α: | 90.01889107° |
| β: | 91.755904° |
| γ: | 89.98766217° |
Sample type: single crystal
Code: FHI-aims
Level of theory: density functional theory
Exchange-correlation functional: HSE06 α = 0.25, ω = 0.11/bohr
Level of relativity: with spin-orbit coupling
Comment: Using the experimental structure of AE4TPbBr4 [1], also in Dataset ID 217. Replace the Br atoms with Cl. Refer to SI Part IX for more details. [1] D. B. Mitzi, K. Chondroudis, and C. R. Kagan, Inorg. Chem. 38, 6246 (1999).
Entry added on: April 15, 2019, 9:54 p.m.
Entry added by: Xiaochen Du Duke University
Last updated on: July 11, 2019, 11:12 p.m.
Last updated by: Xiaochen Du Duke University
Data correctness verified by:
- Rayan C Duke University
Download data
Band structure Verified
See all entries for this property (2 total)
Theory then geometry optimized
Origin: computational
Crystal system:
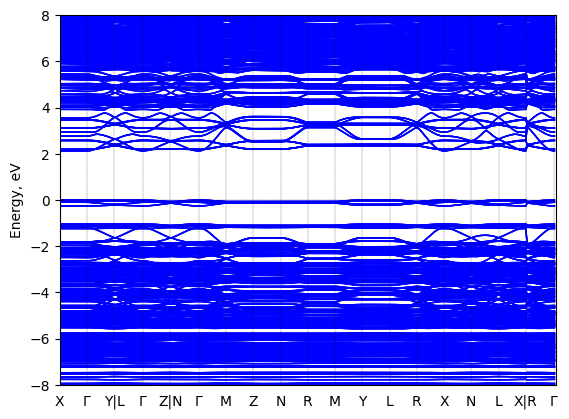
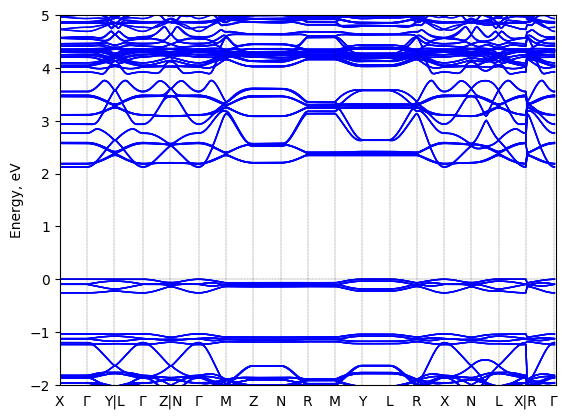
Sample type: single crystal
Code: FHI-aims
Level of theory: density functional theory
Exchange-correlation functional: HSE06 α = 0.25, ω = 0.11/bohr
K-point grid: 3x3x3
Level of relativity: atomic ZORA with spin-orbit coupling
Basis set definition: tight
Entry added on: May 8, 2019, 4:04 p.m.
Entry added by: Xiaochen Du Duke University
Last updated on: May 8, 2019, 4:04 p.m.
Last updated by: Xiaochen Du Duke University
Data correctness verified by:
- Rayan C Duke University
Download data
Absorption spectrum Verified
See all entries for this property (2 total)
Origin: experimental (T = 298.0 K)
Sample type: film
Starting materials: AE4T*2HCl, PbCl2
Product: AE4TPbCl4 film on glass or quartz
Description: Thin film growth by RIR-MAPLE method from a 2 mM solution of the precursor salts using a 1:1 vol:vol blend of DMSO and ethylene glycol as the solvent. Films annealed in nitrogen at 125 C for 5 min after deposition.
Method: UV-vis absorption
Description: UV-vis spectra were collected using a Shimadzu UV-3600 spectrophotometer using a blank substrate as reference.
Entry added on: June 6, 2019, 11:48 p.m.
Entry added by: Wiley Dunlap-Shohl University of Washington
Last updated on: April 9, 2022, 2:45 p.m.
Last updated by: Rayan C Duke University
Data correctness verified by:
- Rayan C Duke University
Download data
Photoluminescence Verified
See all entries for this property (2 total)
Origin: experimental (T = 298.0 K)
Sample type: film
Starting materials: AE4T*2HCl, PbCl2
Product: AE4TPbCl4 film on glass or quartz
Description: Thin film growth by RIR-MAPLE method from a 2 mM solution of the precursor salts using a 1:1 vol:vol blend of DMSO and ethylene glycol as the solvent. Films annealed in nitrogen at 125 C for 5 min after deposition.
Method: Photoluminescence
Description: Steady-state PL spectra were recorded using Edinburgh Instruments FS920 fluorimeter that was equipped with a 450 W xenon arc lamp as the excitation source, and a Peltier-cooled Hamamatsu R2658P photomultiplier tube.
Entry added on: June 6, 2019, 11:57 p.m.
Entry added by: Wiley Dunlap-Shohl University of Washington
Last updated on: April 9, 2022, 3:19 p.m.
Last updated by: Rayan C Duke University
Data correctness verified by:
- Rayan C Duke University
Download data