Bis(phenylethylammonium) lead iodide
Chemical Formula: C16H24N2PbI4
IUPAC: bis(2-phenylethane-1-aminium) lead(II) iodide
Alternate Names: bis(2-phenylethane-1-aminium) tetraiodoplumbate(II), Bisphenylethylammonium lead iodide, phenethylammonium lead iodide, (PEA)2PbI4, PEA2PbI4, (C6H5C2H4NH3)2PbI4, (C8H12N)2PbI4, (C8H9NH3)2PbI4, PEA lead iodide, (PEA)2 lead iodide
Organic: C8H12N
Inorganic: PbI4, Lead iodide
Dimensionality: 2D n: 1
Formal Stoichiometry:
C : 16
,
H : 24
,
N : 2
,
Pb : 1
,
I : 4
Atomic structure
See all entries for this property (31 total)
Method: Single-crystal X-ray diffraction
Origin: experimental (T = 298.0 K)
Space group: P -1
Crystal system: triclinic
| a: | 8.7389 (±0.0002) Å |
| b: | 8.7403 (±0.0002) Å |
| c: | 32.9952 (±0.0006) Å |
| α: | 84.646 (±0.001)° |
| β: | 84.657 (±0.001)° |
| γ: | 89.643 (±0.001)° |
- temperature = 298.0 K
Sample type: single crystal
- data set 745 (atomic structure)
- data set 1885 (atomic structure)
- data set 1901 (atomic structure)
- data set 1903 (atomic structure)
- data set 2267 (band gap (fundamental))
- data set 2269 (band structure)
Starting materials: PbI2 (99.999% trace metal basis), HI (57 wt % in H2O, with hypophosphorous acid as stabilizer, assay 99.95%), CH3OH (>99.9%), 2-phenylethylamine (PEA, 99%)
Product: Red and laminar crystals.
Description: Dissolve PbI2 (54.6 mg) in 0.5 mL of HI (57%). Place CH3OH (1 ml) on the top of the PbI2 solution. Add 0.030 mL of PEA liquid into the CH3OH layer. Crystals would form in the solution overnight.
Method: Single-crystal X-ray diffraction
Description: Single-crystal X-ray diffraction data were collected using a Bruker D8 ADVANCE Series II at room temperature. The crystal structures were solved and refined by Shelxl and Olex software.
Comment: This is the experimentally resolved structure, which contains a statistical (disordered) representation of the equatorial iodine atoms in the original refinement. This structure is our recommended "best" published experimental structure for phenethylammonium lead iodide at room temperature, supported by a computational comparison of the total energies of various published structures after full structure optimization in Chemistry of Materials, Vol. 34, issue 7, 3109-3122 (2022), https://doi.org/10.1021/acs.chemmater.1c04213. The computationally relaxed (PEA)2PbI4 structure with resolved disorder (i.e., no overlapping iodine positions), optimized using the PBE+Tkatchenko-Scheffler approach, is also available in the HybriD3 database (see datasets linked to the present one.)
Entry added on: April 15, 2019, 9:54 p.m.
Entry added by: Xiaochen Du Duke University
Last updated on: Sept. 10, 2024, 4:09 p.m.
Last updated by: Volker Blum Duke University
Download data
Exciton binding energy
See all entries for this property (2 total)
Origin: experimental (T = 293.0 K)
Space group: P-1
Crystal system: triclinic
| Exciton binding energy, eV |
|---|
- temperature = 293.0 K
Sample type: single crystal
- data set 2259 (band gap (fundamental))
- data set 2261 (Exciton energy)
- data set 2266 (Exciton energy)
Starting materials: 200 mg (0.90 mmol) of PbO and 200 μL (1.59 mmol) of phenylethylammonium, fully dissolved in 4 mL of HI and 0.5 mL of H3PO2 solution.
Product: Exfoliated single crystal flakes of (PEA)2PbI4.
Description: 2D perovskite PEPI single crystals are synthesized based on previously reported slow-cooling method in Ref. https://dx.doi.org/10.1021/acsenergylett.8b01315. 200 mg (0.90 mmol) of PbO and 200 μL (1.59 mmol) of phenylethyl- ammonium are fully dissolved in 4 mL of HI and 0.5 mL of H3PO2 solution at 90 °C. The solution is then slowly cooled to room temperature at a rate of 2 °C h−1, giving orange sheet-like crystals. The crystals are then isolated from the parent solution by vacuum filtration, washed by a small amount of diethyl ether, and dried under vacuum. Thin crystals were exfoliated from the parent crystal using stiff heat release tape that serves as a handle. Sequential exfoliation steps with the tape yield successively thinner crystals. Many crystals were surveyed to select the best surface quality, flatness, and area.
Comment: Note that, while an XRD pattern was reported in this work, the XRD analysis and the space group were not reported and the space group listed here was taken from DOI: 10.1021/acs.inorgchem.7b01094.
Method: Reflection mode and transmission mode ellipsometry
Description: Transmittance was collected on a Cary 7000 UV-VIS-NIR spectrophotometer. Reflection ellipsometry was collected on a JA Woollam M2000DI at 45° to 75° using tape to suppress backside reflections. Transmission ellipsometry was collected on a JA Woollam M2000DI from −10° to 70°. The three data sets were processed as a multisample analysis in CompleteEASE. For bulk and cleaved crystals, reflection ellipsometry and reflection Mueller Matrix were collected using focus probes and either a JA Woollam M2000 or RC2, respectively.
Comment: Exciton energies were extracted from a uniaxial model of the ellipsometry data (2.385(5) eV in-plane and 2.419(7) eV out-of-plane). The exciton binding energy of 0.259 eV was calculated using ellipsometry dielectric parameters, an electron-hole image charge model and the experimental effective mass of DOI: 10.1021/acs.jpclett.0c03731.
Entry added on: March 4, 2023, 5:55 p.m.
Entry added by: Volker Blum Duke University
Last updated on: March 6, 2023, 12:02 p.m.
Last updated by: Volker Blum Duke University
Download data
Photoluminescence Verified
See all entries for this property (3 total)
Origin: experimental (T = 298.0 K)
Space group: P -1
Sample type: film
Starting materials: (PEA)2PbI4 crystals, DMF, glass substrates
Product: Thin film on glass
Description: Dissolve the 2D single crystals into DMF at a concentration 6%∼10% relative to the total weight. Spin-coat at 3000 rpm for 30 s on glass substrates. Anneal in air at 100 °C for 10 min before measurement.
Method: Photoluminescence
Description: The photoluminescence spectra were measured using a Horiba-Jobi-Yvon LabRAM ARAMIS system, with a 325 nm He-Cd laser excitation. The laser beam was collimated and focused through a 40X UV objective onto the sample surface at room temperature. Refer to figure 5.
Entry added on: March 14, 2019, 4:18 p.m.
Entry added by: Xiaochen Du Duke University
Last updated on: June 22, 2022, 9:35 p.m.
Last updated by: Rayan C Duke University
Data correctness verified by:
- Rayan C Duke University
Download data
Photoluminescence peak position Verified
Origin: experimental (T = 298.0 K)
Space group: P -1
Crystal system: triclinic
| Photoluminescence peak position, nm |
|---|
- temperature = 298.0 K
Sample type: film
Starting materials: (PEA)2PbI4 crystals, DMF, glass substrates
Product: Thin film on glass
Description: Dissolve the 2D single crystals into DMF at a concentration 6%∼10% relative to the total weight. Spin-coat at 3000 rpm for 30 s on quartz substrates. Anneal in air at 100 °C for 10 min before measurement.
Method: Photoluminescence
Description: The photoluminescence spectra were measured using a Horiba-Jobi-Yvon LabRAM ARAMIS system, with a 325 nm He-Cd laser excitation. The laser beam was collimated and focused through a 40X UV objective onto the sample surface at room temperature. Refer to figure 5.
Entry added on: May 23, 2019, noon
Entry added by: Xiaochen Du Duke University
Last updated on: June 22, 2022, 10:09 p.m.
Last updated by: Rayan C Duke University
Data correctness verified by:
- Rayan C Duke University
Download data
Band structure
Method: DFT-HSE06 (alpha=0.25, omega=(0.11 Bohr radii)^-1)+ SOC
Origin: computational
Crystal system: triclinic
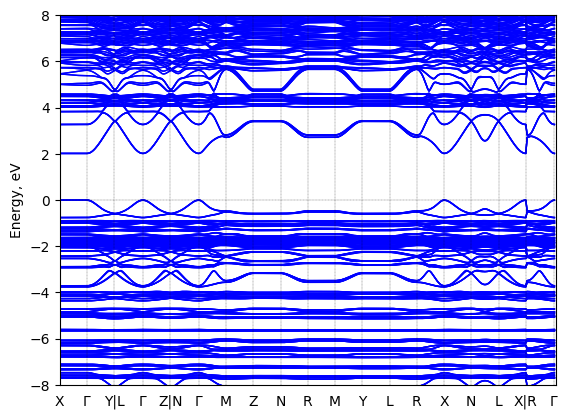
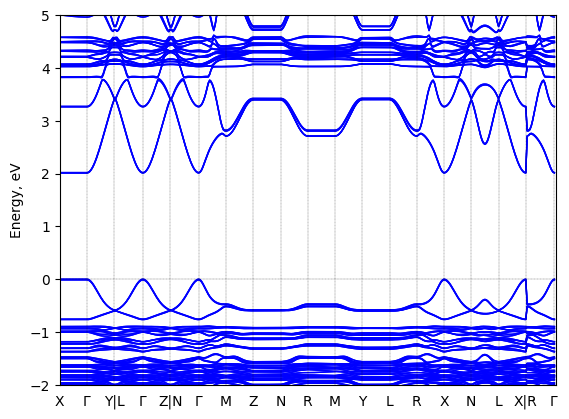
Sample type: single crystal
- data set 225 (atomic structure)
- data set 745 (atomic structure)
- data set 1885 (atomic structure)
- data set 1901 (atomic structure)
- data set 1903 (atomic structure)
- data set 2267 (band gap (fundamental))
Code: FHI-aims
Level of theory: Spin-orbit coupled hybrid DFT
Exchange-correlation functional: HSE06 functional; exchange mixing parameter: 0.25, screening parameter: 0.11 (Bohr radii)^(-1)
K-point grid: 3x7x7
Level of relativity: Spin-orbit coupling included as follows: Self-consistent scalar relativity (atomic zero-order regular approximation) with spin-orbit coupling applied non-selfconsistently in the energy band structure calculation.
Basis set definition: All-electron; "intermediate" numerical settings and basis sets.
Numerical accuracy: Note that DFT-computed energy band gap values, even at the level of DFT-HS06+SOC, are not intended to capture the experimentally correct fundamental gap with quantitative accuracy. Rather, they are collected be comparable to other computational band gaps at the same level of theory in order to capture trends between different sources.
Geometry used in the calculation
Comment: The geometry used was computationally optimized (unit cell and atomic positions) starting from the XRD-determined structure reported in https://doi.org/10.1021/acs.inorgchem.7b01094 . The level of theory used was DFT-PBE including the Tkatchenko-Scheffler van der Waals correction. The structure is available in the HybriD3 database as dataset number 745.
Entry added on: March 7, 2023, 11:32 a.m.
Entry added by: Xixi Qin Duke University
Last updated on: March 7, 2023, 11:35 a.m.
Last updated by: Xixi Qin Duke University
Download data
Exciton energy
See all entries for this property (3 total)
Origin: experimental (T = 293.0 K)
Space group: P-1
Crystal system: triclinic
| Exciton energy, eV |
|---|
- temperature = 293.0 K
Sample type: single crystal
- data set 2259 (band gap (fundamental))
- data set 2260 (exciton binding energy)
- data set 2266 (Exciton energy)
Starting materials: 200 mg (0.90 mmol) of PbO and 200 μL (1.59 mmol) of phenylethylammonium, fully dissolved in 4 mL of HI and 0.5 mL of H3PO2 solution.
Product: Exfoliated single crystal flakes of (PEA)2PbI4.
Description: 2D perovskite PEPI single crystals are synthesized based on previously reported slow-cooling method in Ref. https://dx.doi.org/10.1021/acsenergylett.8b01315. 200 mg (0.90 mmol) of PbO and 200 μL (1.59 mmol) of phenylethyl- ammonium are fully dissolved in 4 mL of HI and 0.5 mL of H3PO2 solution at 90 °C. The solution is then slowly cooled to room temperature at a rate of 2 °C h−1, giving orange sheet-like crystals. The crystals are then isolated from the parent solution by vacuum filtration, washed by a small amount of diethyl ether, and dried under vacuum. Thin crystals were exfoliated from the parent crystal using stiff heat release tape that serves as a handle. Sequential exfoliation steps with the tape yield successively thinner crystals. Many crystals were surveyed to select the best surface quality, flatness, and area.
Comment: Note that, while an XRD pattern was reported in this work, the XRD analysis and the space group were not reported and the space group listed here was taken from DOI: 10.1021/acs.inorgchem.7b01094.
Method: Reflection mode and transmission mode ellipsometry
Description: Transmittance was collected on a Cary 7000 UV-VIS-NIR spectrophotometer. Reflection ellipsometry was collected on a JA Woollam M2000DI at 45° to 75° using tape to suppress backside reflections. Transmission ellipsometry was collected on a JA Woollam M2000DI from −10° to 70°. The three data sets were processed as a multisample analysis in CompleteEASE. For bulk and cleaved crystals, reflection ellipsometry and reflection Mueller Matrix were collected using focus probes and either a JA Woollam M2000 or RC2, respectively.
Comment: Exciton energies were extracted from a uniaxial model of the ellipsometry data (2.385(5) eV in-plane and 2.419(7) eV out-of-plane). The value provide here is extracted from a cleaved crystal, in-plane.
Entry added on: March 4, 2023, 6:01 p.m.
Entry added by: Volker Blum Duke University
Last updated on: March 6, 2023, 12:02 p.m.
Last updated by: Volker Blum Duke University
Download data
Band gap (fundamental)
See all entries for this property (3 total)
Method: Ellipsometry
Origin: experimental (T = 293.0 K)
Space group: P-1
Crystal system: triclinic
| Band gap (fundamental), eV |
|---|
- temperature = 293.0 K
Sample type: single crystal
- data set 2260 (exciton binding energy)
- data set 2261 (Exciton energy)
- data set 2266 (Exciton energy)
Starting materials: 200 mg (0.90 mmol) of PbO and 200 μL (1.59 mmol) of phenylethylammonium, fully dissolved in 4 mL of HI and 0.5 mL of H3PO2 solution.
Product: Exfoliated single crystal flakes of (PEA)2PbI4.
Description: 2D perovskite PEPI single crystals are synthesized based on previously reported slow-cooling method in Ref. https://dx.doi.org/10.1021/acsenergylett.8b01315. 200 mg (0.90 mmol) of PbO and 200 μL (1.59 mmol) of phenylethyl- ammonium are fully dissolved in 4 mL of HI and 0.5 mL of H3PO2 solution at 90 °C. The solution is then slowly cooled to room temperature at a rate of 2 °C h−1, giving orange sheet-like crystals. The crystals are then isolated from the parent solution by vacuum filtration, washed by a small amount of diethyl ether, and dried under vacuum. Thin crystals were exfoliated from the parent crystal using stiff heat release tape that serves as a handle. Sequential exfoliation steps with the tape yield successively thinner crystals. Many crystals were surveyed to select the best surface quality, flatness, and area.
Comment: Note that, while an XRD pattern was reported in this work, the XRD analysis and the space group were not reported and the space group listed here was taken from DOI: 10.1021/acs.inorgchem.7b01094.
Method: Reflection mode and transmission mode ellipsometry
Description: Transmittance was collected on a Cary 7000 UV-VIS-NIR spectrophotometer. Reflection ellipsometry was collected on a JA Woollam M2000DI at 45° to 75° using tape to suppress backside reflections. Transmission ellipsometry was collected on a JA Woollam M2000DI from −10° to 70°. The three data sets were processed as a multisample analysis in CompleteEASE. For bulk and cleaved crystals, reflection ellipsometry and reflection Mueller Matrix were collected using focus probes and either a JA Woollam M2000 or RC2, respectively.
Comment: Exciton energies were extracted from a uniaxial model of the ellipsometry data (2.385(5) eV in-plane and 2.419(7) eV out-of-plane). The exciton binding energy of 0.259 eV was calculated using ellipsometry dielectric parameters, an electron-hole image charge model and the experimental effective mass of DOI: 10.1021/acs.jpclett.0c03731.
Entry added on: March 4, 2023, 5:52 p.m.
Entry added by: Volker Blum Duke University
Last updated on: March 6, 2023, 12:01 p.m.
Last updated by: Volker Blum Duke University
Download data
Absorption spectrum
See all entries for this property (4 total)
Origin: experimental (T = 298.0 K, 298.0 K)
Sample type: film
Starting materials: phenethylammonium iodide (C8H12IN), lead iodide (PbI2)
Product: thin film
Description: (PEA)I, amd PbI2 made up the target solution in 1:1 DMSO/MEG by volume. They were mechanically mixed until visibly dissolved in solvent, taking about 5 min. In a growth chamber, the solution is cooled to -196°C under vacuum. When frozen, the top layer is removed using an Er:YAG laser (2.94 μm). The laser rasters across the surface to sublimate the MEG, causing the precursor material to be ejected onto the substrate (2 cm × 2 cm of SiO2 glass) spinning 7 cm above. The substrate temperature is approximately 10 °C while in the growth chamber. Deposit time was 4 h. Samples remained in a load lock under turbo vacuum (2 × 10–5 Torr) for an hour afterwards. The annealed films were additionally annealed for 10 min on a hot plate in an N2 environment at 110°.
Method: UV-vis absorption
Description: UV−vis absorption spectra were acquired using a Shimadzu UV-3600 spectrophotometer. Samples of films on glass substrates were measured. Samples were kept in ambient air conditions.
Entry added on: Sept. 11, 2022, 10:26 a.m.
Entry added by: Elisa Wade Albert-Ludwigs-Universität Freiburg
Last updated on: Sept. 11, 2022, 10:32 a.m.
Last updated by: Elisa Wade Albert-Ludwigs-Universität Freiburg
Download data
Band gap (optical, transmission) Verified
Origin: experimental (T = 298.0 K)
Space group: Cc
Crystal system: monoclinic
| Band gap (optical, transmission), eV |
|---|
- temperature = 298.0 K
Sample type: film
Starting materials: (PEA)2PbI4 (synthesized), DMF
Description: Thin films were prepared by dissolving (PEA)2PbI4 (0.50M) in DMF and was spin-coated onto glass substrates at 3000 rpm for 30 s. The films were then heated to 130ºC for 10 minutes.
Method: UV-vis absorption
Description: the spectrum was recorded using SHIMADZU UV-3600.
Entry added on: July 23, 2020, 11:18 p.m.
Entry added by: Rebecca Lau Duke University
Last updated on: Sept. 16, 2022, 2:50 p.m.
Last updated by: Rayan C Duke University
Data correctness verified by:
- Rayan C Duke University
Download data
Photoluminescence excitation Verified
Origin: experimental (T = 4.0 K)
Sample type: single crystal
Starting materials: PbI2, BiI3, C6H5C2H4NH3I
Product: dark, red, platelike crystals, with a bismuth ratio Pb/Bi of approx. 0.3%
Description: Under an argon atmosphere, lead iodide (PbI2), bismuth iodide (BiI3) and phenylethylammonium iodide (C6H5C2H4NH3I) were mixed at a molar ratio of 0.9:0.1:2 and heated at 333K for a duration of 30 min. As a solvent, nitromethane was added for crystallization.
Method: PLE-spectroscopy
Description: As a detector, a charge-coupled device (CCD) camera cooled by liquid nitrogen and equipped with a polychromator was utilized. PL spectra were measured with an excitation by Xe-lamp light at 2.48 eV. PLE spectra represent detected PL at 2.35 eV.
Comment: Note that the x axis of PLE spectroscopy is the excitation frequency, which is varied in this experiment, while the y axis is the PL intensity measured at fixed energy (here, 2.35 eV).
Entry added on: March 10, 2022, 1:04 p.m.
Entry added by: Elisa Wade Albert-Ludwigs-Universität Freiburg
Last updated on: March 5, 2023, 1:57 p.m.
Last updated by: Volker Blum Duke University
Data correctness verified by:
- Volker Blum Duke University
Download data
DOI for this data set: 10.1103/PhysRevB.70.205330
Data set ID: 1940 Did you find any mistakes or inconsistencies about this data? Send us a note and we'll have a look at it and send you a reply. Thanks!Stokes shift
Origin: experimental
Space group: C1c1
Crystal system: monoclinic
| Stokes shift, nm |
|---|
Sample type: unknown
Entry added on: July 23, 2020, 11:19 p.m.
Entry added by: Rebecca Lau Duke University
Last updated on: Sept. 16, 2022, 2:59 p.m.
Last updated by: Rayan C Duke University
Download data